CASE REPORT
Carotid EndoVAC: a novel hybrid technique for carotid Dacron patch infection
Chan E,1 Bailey M,1 McPherson S,2 Wanhainen A,3 Scott DJA,1 Forsyth J1
Abstract
This paper reports the use of the EndoVAC technique in a case of left carotid artery Dacron patch infection. The EndoVAC technique involves a sequence of endovascular relining with a stent graft, surgical debridement with explantation of infected graft material and secondary intention wound healing with a vacuum-assisted closure (VAC) device and long-term antibiotic treatment. Our patient was considered a high-risk surgical candidate due to previous cranial nerve injury, high carotid stent graft positioned in zone 3 of the neck, and high stroke risk due to aberrant cerebrovascular anatomy. The carotid EndoVAC technique was performed without complication and a successful outcome was observed at 1-year follow-up. Although currently not recommended as first-line management in treating vascular graft and endograft infections of the carotid vessels, the EndoVAC approach should be considered in a select cohort of patients when neither traditional radical surgery nor conservative simple VAC therapy are considered feasible or safe.
Introduction
Vascular graft and endograft infections (VGIs) are one of the more challenging postoperative complications in vascular surgery. Traditional recommendations for treating VGIs include two extremes of either radical debridement with in situ or extra-anatomical revascularisation, or conservative management with life-long antibiotics and endovascular stent grafts (SG).1 The EndoVAC technique is a hybrid approach to the management of VGI first described in 2011 by Kragsterman et al.2 This paper discusses the use of the EndoVAC technique in treating a challenging carotid Dacron patch infection.
Case report
In 2017, a 75-year-old woman was referred for a left carotid endarterectomy (CEA) for a symptomatic left internal carotid artery (ICA) stenosis. Other medical history included hypertension, polymyalgia rheumatica, Barrett’s oesophagus, hiatus hernia, hyperlipidaemia and osteopenia. A left CEA with Dacron patch was performed under local anaesthesia with conversion to general anaesthetic. Postoperatively, a left recurrent laryngeal nerve palsy was diagnosed, which fully resolved. Three years later she presented with left-sided neck swelling and pain. A carotid duplex showed a linear and hypoechoic area anterior to the left ICA patch which was corrugated, suggesting patch infection (Figure 1).
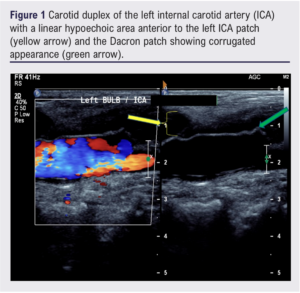
Computed tomography angiography (CTA) suggested an infected collection or thrombosed pseudoaneurysm at the caudal end of the patch. A 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography (18F-FDG PET CT) and ultrasound-guided fine needle aspiration was arranged on an urgent outpatient basis. The following week the patient presented with bleeding from the left neck lump. CTA confirmed anastomotic dehiscence and partially thrombosed pseudoaneurysm.
Emergency endovascular relining of the left ICA was performed with two (6 mm x 25 mm and 5 mm x 40 mm) Viabahn Endoprosthesis SG (W L Gore & Associates Inc, Flagstaff, Arizona, USA). A 7 mm Amplatzer plug (Abbott Medical, Plymouth, Minnesota, USA) was placed into the external carotid artery (ECA) to prevent retrograde flow (Figure 2). She remained on dual antiplatelet therapy to reduce the risk of SG thromboembolism. The patient initially declined surgical explantation and reconstruction. She trialled conservative management with antibiotics, but this failed as she reported new night sweats and recrudescence of the left neck lump.
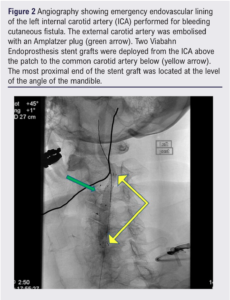
She was considered a high-risk surgical candidate due to a previous history of cranial nerve injury, high carotid SG encroaching on zone III of the neck, incomplete circle of Willis, and a left ICA supplying both the anterior and posterior circulations of the left cerebral hemisphere. Full explantation and autologous vein repair would likely require dislocation of the mandible with no bail-out option to ligate the left carotid system due to an unacceptably high stroke risk.
The EndoVac procedure was considered as per the European Society of Vascular Surgery Guidelines on Vascular Graft and Endograft Infection.3 Endovascular re-lining was performed into the previous SG. New 6 mm x 100 mm and 8 mm x 100 mm Viabahn SG were deployed from the skull base to the left common carotid artery origin. The infected Dacron patch was explanted with minimal debridement (Figure 3). The carotid vessels were not controlled and the underlying carotid SG was left undisturbed. The ECA continued to backbleed, so the Amplatzer plug was removed and the ECA was formally ligated. The exposed vessels and Viabahn SG were partially covered by the overlying sternocleidomastoid muscle. A white (denser) polyurethane foam sponge was placed over the wound, and the VAC was applied and set at a continuous pressure of 125 mmHg. There were no immediate postoperative complications. Dual antiplatelet therapy was continued postoperatively with a plan to consider switching to the COMPASS regime after a few weeks.
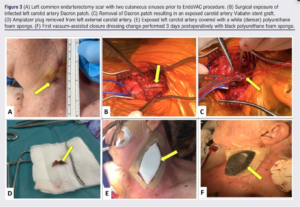
Three days postoperatively, the prosthesis was completely covered with sternocleidomastoid and granulation tissue. Nineteen days postoperatively, the VAC dressing was switched to regular dressings. She was discharged the following day. Five weeks postoperatively the wound was fully healed. She continued on lifelong oral doxycycline (as a suppressive measure). A 18F-FDG PET CT performed 13 weeks post-EndoVAC showed significant improvement in appearances compared with prior studies. One year post-EndoVAC, the patient has clinically improved and her neck wound remains healed.
Discussion
VGIs are recognised as rare but serious life and limb threatening complications of vascular surgery.4 Synthetic patches are advantageous during CEA due to their wide availability, low graft rupture rate and preservation of the saphenous vein. Disadvantages of synthetic patches include suture hole bleeding, pseudoaneurysm and a higher rate of infections. Although synthetic CEA patch infections are rare with a reported incidence of 0.25–0.5%, they remain a recognised complication of CEA with a high morbidity of 29%, ischaemic stroke rate of 5.8% and infection-related mortality rate of 2.3%.1,5 Only 131 cases of carotid patch infection have been reported in the literature.6 Risk factors include poor oral hygiene, long-term steroid usage, immunosuppressants, smoking and diabetes.3
The majority of post-CEA patch infections present with neck swelling, chronic sinus and/or pseudoaneurysm formation, usually >6 months from original surgery.6 In our case, a diagnosis of Dacron patch infection was made on carotid duplex findings of hypoechoic collection and corrugated patch appearance.3 CTA is considered the gold standard investigation modality for VGI. Signs of VGI on CTA include gas, fluid, soft tissue enhancement, pseudoaneurysm and discontinuation of the aneurysmal wall. Although CTA can confirm a diagnosis of VGI, it is often insufficient in isolation and a second imaging modality is recommended with 18F-FDG PET CT or white blood cell scintigraphy (WBCS) combined with single photon emission CT. WBCS identifies areas of increased radiolabelled white blood cells over time, thus showing potential sites of infection.3
The gold standard for treatment of CEA patch infections is radical debridement involving removal of the prosthetic graft and infected tissue with autologous vein revascularisation.4 This approach is associated with stroke and mortality rates of up to 9–12%.7 Revision carotid artery surgery is associated with a high incidence of blood loss, cranial nerve injury (8%), perioperative stroke (6%), recurrent infection (8%) and death (5%).5,8 No agreed consensus regarding the optimal approach to arterial reconstruction exists, although the use of autologous vein patch seems sensible given high re-reinfection rates (50%) with synthetic material.5,7 Ligation of the carotid artery is controversial due to high stroke rates (50%) and mortality, although this may be useful in patients with ICA occlusion.7 Factors against radical debridement with reconstruction include significant comorbidities, unfavourable anatomy and, of course, patient choice. For this cohort of patients, other options include long-term antibiotics, endovascular SG, negative pressure dressings and superficial debridement with or without muscle flap coverage.9 Endovascular SG is an attractive option for those at immediate risk of haemorrhage, although this is balanced with the risk of embolisation, thrombosis and recurrent infection. In isolation, SG is often viewed as a temporising ‘damage control’ measure or a pseudo-palliative approach if no further vascular surgery is planned.
In patients where radical debridement or conservative VAC therapy are not considered options, the EndoVAC technique may be considered in selected patients, albeit supported by low levels of evidence.3 The EndoVAC technique offers a relatively safe method of removing the infected synthetic material with minimal dissection and without arterial clamping. The EndoVAC technique involves three steps: (1) endovascular relining with SG; (2) minimal surgical debridement with explantation of infected graft material, without arterial clamping; and (3) secondary intention wound healing with a VAC device and long-term antibiotic treatment.2,4
Kragsterman et al recommend using continuous negative pressure of 125 mmHg for the first 24 hours followed by intermittent negative pressure.2 The VAC dressing should be changed every 2–4 days. Once the carotid vessels/stent are fully covered by granulation tissue, the white polyurethane foam sponge can be switched to a black polyurethane foam sponge.
Although EndoVAC outcomes have been promising so far, this procedure can of course be criticised for the controversial placement of a synthetic SG within an already infected field. This argument can be rebutted by the accepted use of vascular reconstructions with graft preservation methods with negative pressure dressings and/or muscle flap coverage.9 Similar steps are taken during the accepted management of mycotic aneurysms. The largest European single-centre study reported positive outcomes in using endovascular SG for the management of mycotic aortic aneurysms.10 There are no reports of recurrent infection with the EndoVAC technique, although limited cases have been performed.
Conclusion
This paper highlights the challenges of managing VGIs and reports our experience with the EndoVAC technique to successfully treat a carotid Dacron patch infection in a very challenging case. Although not officially recommended as first-line or gold standard management in treating carotid VGIs, the EndoVAC approach is a viable management option in a highly selected and specific patient cohort. We encourage readers to publish their similar experiences to clarify the indications and long-term outcomes of the EndoVAC technique.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2023;2(3):182-185
Publication date:
May 9, 2023
Author Affiliations:
1. Leeds Vascular Institute, Leeds General Infirmary, Leeds, UK
2. St James University Hospital, Leeds, UK
3. Department of Vascular Surgery, Uppsala University Hospital, Uppsala, Sweden
Corresponding author:
Emily Chan
Vascular Specialty Trainee, Leeds Vascular Institute, Leeds General Infirmary, Leeds, LS1 3EX, UK
Email: [email protected]











