TRIAL PROTOCOL
Does the level of encouragement affect 6-minute walk test performance in patients with intermittent claudication? A protocol for a randomised multicentre controlled trial
Whorlton-Jones ER,1 Seed SA,1 Waddell A,2 Caldow E,3 Harwood AE,2 Birkett ST1
Plain English Summary
Why we are undertaking the research: Peripheral artery disease is a common problem where the blood vessels in the leg are narrowed by fatty build-ups. These fatty deposits may restrict blood flow which causes pain during exercise and limits how far people can walk. To assess a patient’s maximal walking distance, clinicians may use a 6-minute walk test whereby patients are asked to walk as far as possible in six minutes. Currently, encouragement is recommended during the test to ensure the patient walks as far as possible. However, the optimal frequency of this encouragement is still debated and may prove an important factor to guarantee the patient performs to the best of their ability.
What we aim to do: We plan to run an investigation to assess whether the frequency of encouragement delivered by an exercise professional affects how far a patient can walk during a 6-minute walk test. People with peripheral artery disease will be asked to enrol in a 6-minute walk test every week for six weeks. During each of their six tests they will receive standardised encouragement at either 1-minute or 2-minute intervals. Following all the tests, the two groups will have their average maximal walking distances compared. At the end of the study we hope to gain an insight into how often standardised encouragement should be delivered during a 6-minute walk test. We also hope to be able to inform future guidelines of the best way to conduct this highly utilised test in people with peripheral artery disease.
Trial Registration:
ClinicalTrials.gov (NCT04586725)
Introduction
Peripheral artery disease (PAD) is characterised by atherosclerotic lesions of the arteries in the lower limbs, resulting in a reduction of blood flow.1 Globally, it is estimated that 236 million people are living with PAD, with the number of cases increasing by 24% from 2000 to 2010.2,3 A classic symptom of PAD is intermittent claudication (IC), characterised by ischaemic muscle pain precipitated by exertion and relieved by rest.4,5 IC is associated with various comorbidities such as diabetes mellitus, hypertension and dyslipidaemia as well as reductions in physical function, quality of life and balance.4,6,7 Pertinently, symptoms cause patients to stop walking, leading to reductions in walking capacity.
To assess symptomatic responses to an intervention, maximal walking capacity is typically the primary outcome in randomised controlled trials (RCTs).8,9 This involves a patient walking for as long as possible until ischaemic leg symptoms or fatigue prevents them from continuing. This is assessed by treadmill10,11 and corridor exercise testing protocols such as the 6-minute walk test (6MWT);8,12 however, inconsistencies remain in their application.13 The American Thoracic Society provides guidelines for performing a standardised 6MWT including verbal phrases that are conducted every minute. Conversely, when the test is applied in PAD, Montgomery and Gardner14 suggest encouragement every two minutes. Encouragement has been shown to significantly affect maximal walking distance (MWD) by as much as 30 metres in heart failure and respiratory disease populations.15 However, the effect of encouragement on walking performance in people with IC is yet to be investigated.16 Therefore, the primary aim of this trial is to assess the impact of different levels of encouragement on MWD during a 6MWT in patients with IC.
Methods and analysis
The study is a multicentre parallel RCT. Patients will be blinded to the trial aims and randomly allocated to one of two 6MWT groups: encouragement at 1-minute intervals following current guidelines (control) or encouragement at 2-minute intervals, on a 1:1 allocation ratio. Adapted from previously published methods,15 patients will conduct six 6MWTs one week apart with MWD recorded at each visit. All sessions will be supervised by a qualified exercise professional or member of the research team. Data will be reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines.17
Trial setting
The trial will be conducted at four centres in the UK: University of Central Lancashire, Atrium Health in Coventry, Coventry University and the University of Salford. Data will be collected at each site.
Trial registration
The trial was prospectively registered on ClinicalTrials.gov (NCT04586725). Any amendments required to this protocol will seek approvals from the research ethics committee before implementation and will be fully reported in the final trial report.
Trial procedures
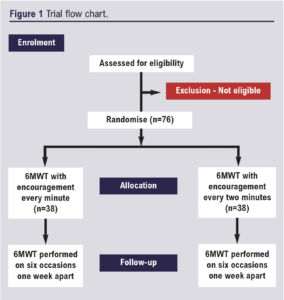
An outline of the patient pathway for the trial is presented in the trial flow chart (Figure 1). Patients will be made aware of the trial at an exercise programme or vascular clinic. Recruitment will be either prior to SEP entry or on completion of the programme depending on site. If they wish, patients will receive a paper copy of the participant information sheet from a clinical or exercise professional. The contact details of the research team will be on the information sheet; therefore, it is the patient’s choice to contact the research team. Once confirmed, patients will be contacted by a member of the research team to determine eligibility. Informed consent will be obtained at the baseline assessment/first 6MWT visit. Eligible participants will subsequently be randomised to the following groups: encouragement at 1-minute intervals or encouragement at 2-minute intervals. Clinical examination will take place prior to the first 6MWT visit and will include a review of medical history, current medications, stature (cm), body mass (kg) and cardiovascular risk factor assessment (resting blood pressure, resting heart rate and smoking status). At all subsequent visits and prior to testing, participants will be verbally screened against the exclusion criteria.
Inclusion criteria
1. Patients recently diagnosed with IC who have been screened by a vascular specialist nurse, clinician or podiatrist will be eligible.
2. >18 years of age.
3. Resting ankle brachial pressure index (ABPI) <0.9 or a reduction of >20 mmHg following exercise testing.
4. Able to walk unaided.
5. English speaking and able to follow exercise instructions.
6. Able to provide informed consent.
Exclusion criteria
1. Those who have critical limb-threatening ischaemia (rest pain and/or tissue loss).
2. Unable to provide consent.
3. Those presenting with any significant comorbidities or contraindications to exercise testing or training in accordance with the American College of Sports Medicine.18
Intervention
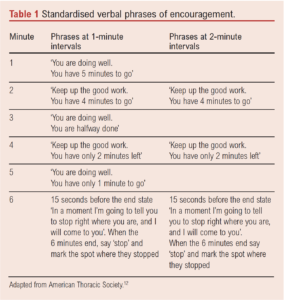
All 6MWTs will be conducted in accordance with American Thoracic Society guidelines with the frequency of encouragement the only deviation.12 In brief, the walking test will be conducted over a 30 metre (100 ft) flat enclosed corridor, with patients instructed to walk as far as possible within 6 minutes. Prior to each test, patients will rest in a chair at a quiet location for at least 10 minutes. A qualified, experienced exercise professional will conduct the tests and deliver standardised verbal phrases of encouragement. These will be delivered at the allocated time points during the test (Table 1). Patients are allowed to rest during the test, but the clock will continue to run and the phrases will be delivered at the allocated time points. Total metres walked will be calculated at the end of each test. To be regarded as having sufficiently adhered to protocol, patients must attend on six occasions, one week apart, and complete all six tests. To verify the safety of the 6MWT, adverse and serious adverse events will be carefully monitored, recorded and reported in line with the principles of Good Clinical Practice (GCP).
Randomisation and blinding
The random allocation sequence of patients will be generated by a trial statistician on a 1:1 basis using a computer program random number generator (https://www.studyrandomizer.com/). To ensure allocation concealment, researchers will request randomisation from the chief investigator on completion of all baseline assessments using a sealed, opaque, sequentially numbered envelope. The concealment will not be created by the researchers who recruit. Researchers and clinicians conducting the assessments will not be blinded; however, they will not be involved in data analysis. The trial statistician will be blinded to the interventions. Under no circumstances will the trial statistician be unblinded.
Outcome measures
The primary outcome is to assess the impact of the frequency of encouragement on MWD performance (difference in metres walked) across six 6MWTs. The secondary outcome is to assess the learning effect of the 6MWT (metres) across six tests, one week apart.
Sample size
Based on recently published work, a minimal clinical important difference of 46 metres16 and standard deviation of 61 metres resulted in an effect size of 0.754. With a power of 80% and a significance level of 5%, a sample size of 58 patients will be recruited would be needed to attain statistical significance. A drop-out of approximately 30% will be allowed, yielding a required sample size of 76 patients to be randomised into the two groups.
Data collection and management
Data will be collected and retained in accordance with the Data Protection Act 2018. The protocol and subsequent trial will adhere to the Standard Protocol Items: Recommendations for Clinical Trials (SPIRIT) and adopts the SPIRIT checklist.19 Trial data will be collected on a case report form by the research team at each 6MWT visit. All patients will be given a study code along with the abbreviated hospital title to ensure anonymity. The anonymised data will be stored using a password protected file on the University of Central Lancashire staff OneDrive system and processed using an institutional Surface Pro. Only named investigators will have access to the patient data. Study documents (paper and electronic) will be retained in a secure location during and after the trial has finished for a minimum period of 5 years.
Data analysis
To examine differences in metres walked between the two encouragement groups at each of the six time points, linear mixed models will be used with ‘group’ modelled as a fixed factor and random intercepts by participants. Furthermore, to examine differences in walking performance between the six time points, repeated measures linear mixed models will be used for each group, with ‘time’ modelled as a fixed factor and random intercepts by participants. Assumptions of normality will be assessed by a Kolmogorov–Smirnov test. Skewness and kurtosis will be visually examined. All analyses will be conducted using SPSS v27 (IBM, New York, USA). For linear mixed models, the mean difference (b), t-value and 95% confidence intervals of the difference will be presented and statistical significance for all analyses is accepted as p<0.05. All data will be summarised and reported in accordance with the CONSORT guidelines.17
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2022;1(4):137-140
Publication date:
May 16, 2022
Author Affiliations:
1. School of Sport and Health Sciences, University of Central Lancashire, Preston, UK
2. Centre of Sports, Exercise and Life Sciences, Institute of Health and Wellbeing, Coventry University, Coventry, UK
3. School of Health and Society, University of Salford, Salford, UK
Corresponding author:
Dr Stefan T Birkett
School of Sport and Health Sciences, University of Central Lancashire, Preston, PR1 2HE, UK
Email: [email protected]











