EDITORIALS
Frailty in peripheral arterial disease
Welsh SA,1,2 Martin P,3 Pathmanathan S,4 Hussey K,2 Brittenden J,1,2 Orr DJ,1,2 Quinn T1
Introduction
It has been consistently demonstrated that frail vascular patients have poorer outcomes compared with their robust counterparts.1 Consideration of frailty is particularly important, not only as our population continues to age but as advances in anaesthetic, surgical and endovascular techniques are enabling a broader range of interventional options for those people who may have traditionally been labelled as unsuitable for surgery.2
Frailty has implications for health and social care at the micro and macro level, and failure to consider the differing needs and natural histories of people living with frailty could result in avoidable harm and suboptimal resource allocation. National guidelines recognise the growing urgency in the need to recognise and manage frailty, yet standardised methods for this have not been identified and agreed upon.3 Despite undeniable prognostic value, the aetiology and pathophysiology of frailty in peripheral arterial disease (PAD) remains poorly understood, albeit new data are emerging. This has challenged a uniform approach to assessment and management.
This editorial considers the theories of frailty and applies these to the assessment and management of patients with peripheral arterial disease.
Frailty: definition and theories
Frailty may be defined as a syndrome of increased vulnerability to external stressors due to a failure in homeostatic reserves brought about by age-associated deficits accrued across multiple domains. Two main underpinning theories are accepted which are not mutually exclusive: the phenotypic and cumulative deficit models. The Fried phenotype of frailty is characterised by progressive age-related deterioration in underlying physical substrate.4 It is defined by the presence of three or more energy-negative components existing in a self-perpetuating cycle: unintentional weight loss, weakness, exhaustion and reduced walking speed and activity levels. Rockwood’s cumulative deficit theory describes a multifactorial and dynamic biological construct where frailty is graded by the progressive accrual of multi-domain deficits.5 Deficits can be quantified to create a Frailty Index (FI) through summation of the number of deficits from a predefined list.
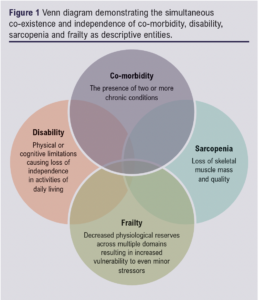
Frailty is related to, but not synonymous with, concepts of comorbidity, disability and sarcopenia (Figure 1). Comorbidity describes the coexistence of two or more chronic conditions. Approximately 70% of frail patients are comorbid, yet only 20% of comorbid patients are frail.6 Disability refers to a limitation in physical or cognitive ability to perform activities of daily living independently,7 whereas frailty describes a vulnerable state at increased risk of developing disability.4,8 Lastly, sarcopenia is a biological syndrome characterised by generalised and progressive loss of skeletal muscle mass and quality.9 Comparing sarcopenia and frailty according to Fried’s definition, low grip strength and slow gait speed are characteristic of both constructs, while low activity levels and weight loss are physical manifestations of frailty and aetiological risk factors for sarcopenia.10
Biology of frailty
Frailty can be considered an accelerated or unsuccessful ageing process which occurs either primarily due to intrinsic dysregulation of homeostatic pathways or secondary to physiological burden imposed by diseases and/or their treatment. Several ageing hallmarks have been defined, including altered intercellular communication, mitochondrial dysfunction, genomic instability, telomere shortening and epigenetic changes.11 Underpinning these age-related changes are chronic inflammation and oxidative stress (Figure 2).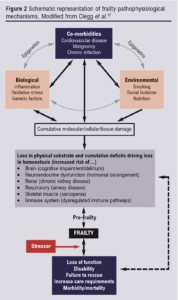
Chronic inflammation
‘Inflammaging’ describes an age-related, chronic, sterile increase and dysregulation of inflammatory processes. Most studies demonstrate significant relationships between frailty and (pro-) inflammatory biomarkers of C-reactive protein and interleukin-6.12 To a lesser extent, elevated levels of clotting pathways constituents (D-dimer, Factor 8 and fibrinogen), tumour necrosis factor-α and total white cells, or reduced levels of haemoglobin and haematocrit are also correlated with frailty, even after correcting for factors like age, sex, smoking status, comorbidities and medications.13,14
Oxidative stress
Frailty has been associated with higher oxidative stress levels and possibly lower antioxidant levels.15,16 This theory describes a cumulative burden of reactive oxygen species causing gradual cell damage, loss of recovery/regeneration and apoptosis or cellular senescence resulting in tissue deterioration and organ dysfunction.17,18 Depending on the tissue/organ affected, different effects are felt (eg, CNS involvement could cause cognitive decline or disrupt neuroendocrine function19-21). A role for oxidative stress in age-related conditions of the kidney and cardiovascular disease has also been demonstrated.22 Once these effects reach a threshold across multiple organ systems, the person becomes clinically frail.
Environmental exposure
Extrinsic exposures have been proposed as initiators/drivers of frailty. Among several factors correlated with frailty are environmental (lower income, social isolation), clinical (comorbidities, poor self-rated health) and ‘lifestyle’ factors (smoking, alcohol, diet).23-28
Epidemiology of frailty in PAD
Approximately 10% of the general population aged over 65 years are frail.29 The estimated prevalence in patients with PAD is 20–60%.30 The greater prevalence and implications in patients with PAD may be driven by shared aetiological risk factors and possibly synergistic biological mechanisms.31-33 Chronic inflammation, increased oxidative stress and shared risk factors (eg, smoking and obesity) are associated with both PAD and frailty.32,33 A bidirectional relationship is apparent as PAD is an independent risk factor for frailty and vice versa.23 This high prevalence of frailty is seen in other vascular diseases such as stroke.34 While heterogeneity in frailty assessment challenges accurate epidemiological reporting, it is clear that global population ageing will result in an increased prevalence of frailty in surgical populations.35
Implications of frailty in PAD
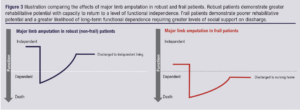
Considered ‘the most problematic expression of population ageing’,17 frailty diminishes resilience to physiological insults, impairs recovery and complicates return to pre-morbid functional levels (Figure 3). In open surgery and endovascular techniques alike,37 frailty significantly increases risk of morbidity, short- and long-term mortality, prolonged hospital admission, discharge with greater social care requirements and functional decline (Figure 4).1,30,36
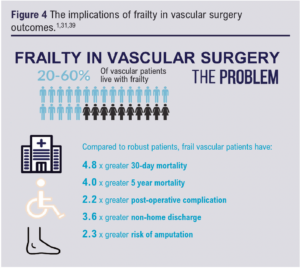
While there is a paucity of health economic data specific to frailty and vascular surgery, undifferentiated economic data demonstrate that, after controlling for confounding factors, frailty significantly increases total healthcare costs by 54–290%.38,39 Frail patients are more likely to have greater social care requirements with estimations suggesting a 16-fold increase in care costs.22 In-hospital costs for frail elective surgical patients are also substantially higher than for their robust counterparts.40
Given these pressures, maintaining the status quo is not an option. Many vascular services are looking at innovative clinical pathways facilitating frailty identification and management (Figure 5).

Assessment of frailty in PAD
The first step in managing frailty is its identification. A recent review confirming the prognostic value of frailty in vascular patients identified the use of 16 different frailty assessment tools.37 Broadly, such tools have been categorised into phenotypic, cumulative deficit/FI and ‘other’ measurements.37 Phenotypic measurements included grip strength, gait velocity, timed-up-and-go test and wearable sensor technology.41–46 Importantly, the majority of vascular-themed frailty research involves patients undergoing lower limb revascularisation.37 Ischaemic rest pain will impair mobility, which may skew results if solely relying on phenotypic measurements. However, end stage arterial disease is systemic, with a propensity to produce critical comorbidities. It remains to be clarified if this ‘skew’ represents a bias in assessment or, in fact, a true estimation of frailty. The original FI incorporated 70 items, reflecting themes included in a comprehensive geriatric assessment (CGA).5 However, this is burdensome for practical clinical application, so more concise iterations have been produced. Expected standards of an FI are to include at least 30 multi-domain variables that have an association with health status and increase in prevalence with age without premature saturation.47 Nine different FIs have been identified in vascular surgery research, and all demonstrate predictive validity despite none conforming with these expectations (all <30 variables).37 Novel frailty markers include nursing home residency,48 biomarkers,49-51 nutritional52 and isolated functional assessments.53-55 The use of radiologically-detected sarcopenia as a frailty marker has also been investigated.56 Standardisation in frailty assessment will benefit research and clinical practice by improving comparison of services, facilitating data pooling and enhancing translatability of results into clinical practice.
Frailty assessment may allow improvements in healthcare including improved prognostication and targeted optimisation of frailty-related domain deficits. First, the prognostic value of several frailty assessment tools has been demonstrated. Tools that are reproducible and quick without requiring additional training or equipment are likely to be more rapidly adopted. One example is the Clinical Frailty Scale (CFS), a 9-point person-assessed tool applicable in less than 1 minute.5 Incorporating frailty assessment into preoperative decision-making may guide patient-centred management. Electively, frailty assessment may be of particular use when considering the suitability of offering prophylactic surgical treatments (eg, carotid endarterectomy) while, in urgent settings, frailty scores may aid decision-making around a patients’ suitability for invasive treatment compared with palliative measures (e.g. in patients with chronic limb-threatening ischaemia). To inform targeted frailty-related optimisation, more exhaustive methods should be considered such as those seen with CGA. The maximum benefit of this assessment is only achieved when applied by an experienced healthcare professional in the correct setting with access to the necessary means and time to implement relevant changes. Incorporating this into a vascular surgery practice mandates establishing service models with regular interdisciplinary contribution from geriatricians, pharmacists, physiotherapists and occupational therapists, complemented by communication with social support services and community follow-up links. To ensure such a resource-intensive service is directed appropriately, it may prove valuable to operate a two-stage system with early frailty screening using a tool such as the CFS, to identify and select those most likely to benefit from CGA.
Frailty management in patients with PAD
Frailty exists along a spectrum of severity with potential for bidirectional transitions between ageing successfully and vulnerability. At a societal level, public health responses to ameliorate frailty effects through health promotion, education and improving access to healthy interventions will drive the biggest benefit to population health. However, even if successful preventative measures are implemented, societal demographic changes suggest frailty will still be prevalent in secondary care, and so it remains imperative that pathways are adjusted accordingly.
The British Geriatrics Society (BGS) has published the ‘Silver Book’, a best practice guidance on addressing the care requirements of older people living with frailty during the first 72 hours of unscheduled medical and surgical admissions.57 Specific to surgery are the BGS and Centre for Perioperative Care guidelines on Perioperative Care for People Living with Frailty.58 Briefly, the guidelines recommend a multidisciplinary holistic approach across primary, secondary and social care. This incorporates preoperative risk assessment and optimisation, lifestyle modification, optimised intraoperative techniques, appropriate postoperative rehabilitation and proactive discharge planning that incorporates links to community and primary care follow-up. There will be inherent challenges in overcoming anticipated issues of funding, resource availability and coordinating the synchronisation of such a multidisciplinary approach across all components of health and social care.
In vascular surgery, the importance of frailty has also been recognised through national guidelines advocating patients have access to CGA.3 However, there remains a paucity of research to inform evidence-based guidelines. Preliminary studies confirm clinical and financial advantages to incorporating CGA in perioperative pathways for vascular surgery patients. For example, a randomised controlled trial (n=209) confirmed clinical and cost-effectiveness of introducing preoperative CGA-based prehabilitation compared to standard care for patients undergoing elective arterial reconstruction.59 This benefit was primarily through reductions in length of stay, but also due to reductions in intensive care use, postoperative clinical reviews, care packages and community rehabilitation referrals. Importantly, a challenge specific to vascular surgery is the time-sensitive nature of a significant proportion of presentations, which precludes meaningful prehabilitation. The use of frailty assessments to guide postoperative involvement of geriatric services for emergency vascular admissions has also demonstrated similar positive effects, with reductions in length of stay and improved short-term readmission rates.60 Large multicentre and long-term follow-up studies are required to substantiate this evidence and support clinicians and policy makers in driving forward suggested augmentations to clinical service models.
Conclusion
While there is debate around the biology of frailty, there is no debate that frailty is common and associated with poor surgical outcomes. Multidisciplinary approaches to assessment and management in vascular surgery have been identified as a priority.58 However, there is a lack of robust evidence to support frailty-centric adaptations to services. A collaborative approach between researchers, multidisciplinary clinicians and policy makers is needed to ensure high-quality, person-centred care for this growing surgical population.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2023;2(3):128-133
Publication date:
May 5, 2023
Author Affiliations:
1. College of Medical, Veterinary and Life Sciences, University of Glasgow, Scotland, UK
2. Department of Vascular Surgery, Queen Elizabeth University Hospital, NHS Greater Glasgow & Clyde, UK
3. Perioperative Frailty and Medicine for the Elderly, NHS Tayside, UK
4. Department of Vascular Surgery, Hull Royal Infirmary, Hull University Teaching Hospitals NHS Trust, Hull, UK
Corresponding author:
Silje Welsh
School of Cardiovascular and Metabolic Health, College of Medical, Veterinary and Life Sciences, University of Glasgow, Lister Building, Glasgow Royal Infirmary, 84 Castle Street, Glasgow G4 0SF, UK
Email: [email protected]











