ORIGINAL RESEARCH
Modern Practice of Diabetic Foot Sampling, Protocols, Pathways, Treatments and Techniques: an audit of specimen transport time from theatre to laboratory for diabetic foot tissue (MODAMP 2)
Condie N,1,5 Murray A,2,5 Travers H,2,5 Jones S,3,5 Moses J,1,5 Srinivasamurthy D,4,5 Cheng S,2,5 Rehman S,3,5 Wall ML1,5
Plain English Summary
Why we undertook the work: Patients with diabetic foot infections who undergo an amputation of a toe or leg have an increased risk of death. Treating infections that may result in an amputation promptly and effectively is essential. Guidelines state that, when patients present with infections of the bone in their lower limb, it is essential any samples taken are processed by the laboratory within 2 hours. If this target time is not met, the microbiology results from the samples taken may not be accurate. This could result in a delay in the correct antibiotics and a delayed discharge from hospital. We aimed to see if this 2-hour target time was being met within hospitals in the Vascular Research Innovation Consortium (VaRICS).
What we did: Four hospitals within VaRICS collected data between December 2021 and February 2022. Included in this data was information on the procedure the patient had, the time any samples were taken from the patient, the way these samples were transported and the time they were received in the laboratory.
What we found: The majority of patients undergoing a procedure did have samples taken. However, less than half of these samples were processed within the recommended time frame of 2 hours.
What this means: Increased awareness is required within theatres and by microbiology laboratory technicians regarding the importance of processing samples correctly.
Abstract
Introduction: Outcomes for patients undergoing minor or major amputations as a result of diabetic foot infections are poor. It is essential that specimens taken during investigations for osteomyelitis in these patients are processed correctly. A failure to do this may result in delays in time to start targeted antibiotic therapy and discharge from hospital.
Methods: Four hospitals in the West Midlands collected retrospective data on diabetic foot amputations between December 2021 and February 2022. Data on the procedure, specimen collection time, transport medium and specimen receipt time were recorded. The data were analysed to see if they met the recommended standards from the United Kingdom Standards for Microbiology Investigations, which recommends that all specimens taken during the investigation for osteomyelitis should be processed within 2 hours.
Results: A total of 129 procedures were performed and 94 of these had specimens taken. Twenty-one specimens (22%) reached the laboratory for processing within the 2-hour recommendation; 15 (29%) of the samples were taken ‘in hours’ and six (14%) were taken ‘out of hours’. Fifty-seven samples had the method of transport recorded, of which 54 (95%) were transported in a dry medium.
Conclusions: Improvement is needed to minimise the time samples take to get from theatre to the laboratory. Increased awareness within theatres and microbiology laboratories about the importance of transporting and processing specimens quickly is required, with the result being a quicker diagnosis of osteomyelitis, accurate antibiotic treatment and overall better patient outcomes.
Introduction
The number of amputations secondary to diabetic foot infections continues to rise in the UK, with more than 185 diabetes-related amputations being carried out per week.1 The 5-year mortality rates of patients with diabetic foot ulceration, minor or major lower limb amputations as a result of diabetic foot infections are 30.5%, 46.2% and 56.6%, respectively.2 It is essential that we treat patients who present with this condition quickly and effectively. The UK Standards for Microbiology Investigations, published by Public Health England in January 2016, recommend that, where osteomyelitis is suspected, bone or soft tissue specimens taken from diabetic foot procedures should be processed within 2 hours to maximise chances of culturing causative organisms.3
Failure to achieve accurate microbiological sampling may result in delays to starting targeted antibiotic therapy, increased risk of recurrence and spread of infection and consequent higher risk of amputation with its associated morbidity and mortality. A recent review of diabetes and lower limb complications reported that specimens were sent from 50% of diabetic foot ulcer debridements, but that only 18% of cases of processed samples influenced antibiotic therapy.4 We aimed to investigate whether vascular networks within the Vascular Research Innovation Consortium (VaRICS) collected, transported and processed diabetic foot specimens within the 2-hour guideline.2
Methods
Four vascular networks (Black Country Vascular Network, Worcestershire Acute Hospitals NHS Trust, University Hospitals Birmingham and Shrewsbury and Telford Hospital NHS Trust) in the VaRICS were involved in this audit. The aim of the audit was to compare the sample rates against the published gold standard – the UK Standards for Microbiology Investigations – published by Public Health England in January 2016.3 Retrospective data on diabetic patients undergoing minor amputations between December 2021 and February 2022 were collated using an Excel proforma, with fields agreed by the leads for the data collection at the networks involved. These data were collected from paper and electronic patient notes, theatre records and electronic reporting systems. The data points collected were the date and time of the procedure, specimen collection time from theatre, specimen type (bone or tissue), transport medium and pathology receipt time. The time to processing was calculated as the time the operation ended to the time the laboratory reported receiving the specimen. The data were sent anonymously between sites using NHSmail and were analysed using descriptive statistics.
Results
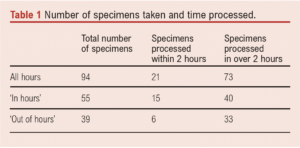
A total of 129 procedures were performed during 3 months of data collection. Specimens were sent from 94 (73%) procedures. Of the 94 specimens taken, 21 (22%) reached the laboratory for processing in <2 hours with a mean time of 10.5 hours. Fifteen (29%) samples taken ‘in hours’ were processed in <2 hours, with a mean time of 7.51 hours, and six (14%) samples taken ‘out of hours’ were processed in <2 hours with a mean time of 14.4 hours. Table 1 shows the number of specimens taken and the time taken for processing.
The transport medium or lack of transport medium was recorded in 54 (61%) samples; 78% of bone and tissue samples were transported in no medium. In the remaining samples the transport medium was either not collected or a beaded (n=1) or amines (n=2) transport medium was used.
Discussion
Overall, we can see that, compared with the recent report on diabetes and lower limb complications, the West Midlands theatre sampling has a higher specimen collection rate than the published literature (77% compared with 50%).4 However, the number of samples processed within 2 hours is only 42%. Out of hours, this is likely due to insufficient staffing to transport the samples and receive them at the laboratory to commence processing. In hours, the gold standard remains for all samples to reach the laboratory within the 2-hour window to start processing.3 This means increased awareness within theatres and by microbiology laboratory technicians about the importance of processing specimens and pre-emptively alerting the team during the theatre briefing.
The guidelines of the UK Standards for Microbiology Investigations advise that samples collected in theatres should be placed in a sterile leak-proof container with Ringer’s or saline solution and then into a sealed plastic bag.3 None of the samples taken in this study were processed in this way, demonstrating a need for education in this area.
To standardise this measurement of time within the study, we used the end of the operation as the specimen collection time. This is a limitation of the study design. This study was carried out in multiple networks, and it was not possible to identify storage conditions of the samples prior to transportation to the laboratory. This may affect the quality of the samples upon arrival at the laboratory, but would be an interesting avenue for further investigation. The National Institute for Health and Care Excellence (NICE) guidance states that, in patients with suspected diabetic foot infections, microbiology samples should be taken to aid with diagnosis and treatment.3 We do not know exactly how taking these samples affects the duration of antibiotic treatment. This is an area of research still to be undertaken.
Conclusion
The Society of Vascular Surgery states that “In patients at high risk for diabetic foot osteomyelitis, we recommend that the diagnosis is most definitively established by the combined findings on bone culture and histology”.5 Ensuring our samples are processed correctly will lead to a quicker diagnosis of osteomyelitis, accurate antibiotic treatment and better patient outcomes. The recent James Lind Alliance exercise put accurate diabetic foot sampling and its consequent treatments at points 2 and 4 on its priority list,6 whilst the recent NHS Resolution document emphasises the importance of getting it right first time with diabetic foot disease as the costs of problems that develop are not inconsequential to the NHS and must be considered.4

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2023;2(4):205-207
Publication date:
July 13, 2023
Author Affiliations:
1. Department of Vascular Surgery, Black Country Vascular Network, Dudley, UK
2. Department of Vascular Surgery, University Hospitals Birmingham, Birmingham, UK
3. Department of Vascular Surgery, Shrewsbury and Telford Hospital NHS Trust, Shrewsbury, UK
4. Department of Vascular Surgery, University Hospitals Coventry and Warwickshire, Coventry, UK
5. Vascular Research Innovation Consortium (VaRICS)
Corresponding author:
Natalie Condie
Department of Vascular Surgery, Black Country Vascular Network, Russells Hall Hospital, Pensnett Road, Dudley DY1 2HQ, UK
Email: nataliecondie@ doctors.org.uk











