ORIGINAL RESEARCH
Symptoms to surgery: factors associated with delays to carotid endarterectomy for symptomatic stenosis in an Irish tertiary vascular centre
Foley MP,1 Gray C,1 Healy D,1 Mulkern E,1 McDonnell CO,1 O’Donohoe MK1
Plain English Summary
Why we undertook the work: Carotid endarterectomy (CEA) is a surgery performed to reduce the risk of stroke by clearing clot-causing debris out of one of the main arteries responsible for bringing blood to the brain. The timing of CEA is critical in patients who have already had a stroke or a mini-stroke. The best evidence indicates CEA should be done within two weeks to minimise the risk of patients going on to have another, potentially larger, stroke. However, getting the surgery done inside this short window is difficult for multiple reasons.
What we did: We reviewed the electronic medical records of consecutive patients who underwent CEA at our hospital after having a stroke or a mini-stroke between January 2017 and December 2019. We looked at how many patients had their surgery within two weeks of their symptoms starting, and at the reasons why some surgeries got delayed.
What we found: One-hundred and twenty-four individual patients were included in the study. We found that just over half of all patients had their surgery within 14 days of their first symptom. Patients who experienced typical stroke signs, as described in the ‘Face Arms Speech Time’ (FAST) mnemonic, were more likely to have their surgery in time; this is largely because they came to hospital sooner. Furthermore, patients who came directly to our hospital, compared with smaller district hospitals without vascular surgeons, were more likely to have their surgery on time.
What this means: Our study shows the number of patients getting their CEA within 14 days at our hospital is comparable to national figures from the UK and Australia. Despite this, a significant number of patients waited over 14 days for surgery. To improve access to timely surgery, systems between hospitals need to be put in place to prioritise these patients. The 14-day target should be mandated as part of the national stroke management strategy.
Abstract
Introduction: Carotid endarterectomy (CEA) is the accepted treatment for stroke risk reduction in patients with symptomatic 50–99% carotid artery stenosis. Best practice guidelines recommend surgery be performed within 14 days of index symptoms to achieve maximum benefit; however, achieving this target can be difficult. Late presentation and presentation to hospitals without onsite vascular capabilities are two recurring reasons for delayed CEA across the literature.
Methods: All symptomatic CEAs performed between January 2017 and December 2019 were retrospectively extracted from an electronic institutional database and analysed to determine the proportion of CEAs meeting benchmark targets and to assess factors contributing to delays.
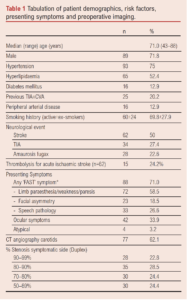
Results: A total of 124 CEAs were performed for 62 strokes and 62 transient ischaemic attacks (TIAs); 71.8% (n=89) were male and the median (range) age was 71.0 years (43–88). The median delay between initial symptom and CEA was 14 days (range 1–183). Sixty-six patients (55%) had surgery within 14 days of the index symptom. Patients presenting with ‘Face Arms Speech Time’ (FAST) symptoms were significantly more likely to undergo timely CEA, largely due to earlier presentation to hospital (p=0.021, OR 2.563, 95% CI 1.143 to 5.747). Presentation directly to a tertiary vascular centre was significantly associated with CEA within 14 days compared with referrals from external hospitals (p=0.002), with a median symptom-to-surgery interval of 12.0 days and 25.0 days, respectively (p=0.001)
Conclusions: Our results are comparable to UK and Australian national audits, although a significant number of CEAs fell outside the 14-day target. Protected pathways between referring hospitals and vascular centres are needed to ensure all patients have equal access to urgent CEA. Designating CEA within 14 days as a key performance indicator for stroke management will hopefully incentivise hospitals to expedite this high-risk cohort. Furthermore, re-auditing with an emphasis on patients turned down for intervention due to recurrent events while awaiting CEA may further highlight the need to prioritise symptomatic carotids.
Introduction
Carotid endarterectomy (CEA) for symptomatic stenosis is most effective when performed close to the index event.1,2 To reduce the risk of further neurological events, the best practice guidelines issued by several international vascular societies all recommend that CEA be performed within 14 days of the initial symptoms.3,4 However, meeting this target can prove difficult without dedicated resources.5-8 Multiple studies have shown that later presentation to an acute hospital and increased numbers of medical practitioners involved in the patient journey cause delays between symptoms and surgery.9,10 Conversely, hospitals with streamlined referral pathways or transient ischaemic attack (TIA) clinics with protected access to diagnostic imaging and vascular surgeons consistently report shorter intervals from presentation to CEA.11,14
At present in Ireland there are no national guidelines enforcing the timing of CEA for symptomatic stenosis; CEA within 14 days of an ischaemic event is not an audited key performance indicator (KPI) for stroke management and vascular surgery procedural data is not recorded in a centralised database. As such, the available data on how Irish patients with symptomatic carotid disease compare with international best practice is limited. Based on published figures from two other urban vascular units, we estimate 50–67% of Irish patients with symptomatic carotid disease undergo CEA within 14 days.15,16 This is comparable to 60% of cases meeting the target reported in the UK National Vascular Registry 2019 Annual Report and 56% in the 2018 Australasian Vascular Audit Public Report.17,18
We report a three-year review of all symptomatic CEAs performed at a single urban university hospital with a large catchment area for tertiary vascular referrals. The aim was to determine how many patients met the benchmark 14-day target for CEA and to determine factors contributing to delays.
Methods
This was a single-centre retrospective case series of consecutive CEAs for symptomatic stenosis at a university-affiliated tertiary referral hospital between 1 January 2017 and 31 December 2019. During the study period, 192 CEAs were performed. All asymptomatic cases (n=66) and two symptomatic cases who were inpatients at the time of the index event were excluded. In total, the study included 124 patients.
Symptomatic carotid artery stenosis has previously been defined by the Society for Vascular Surgery’s reporting standards for carotid interventions.19 The degree of stenosis was determined using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) duplex criteria, where 50–99% was considered significant.20 The decision to operate was largely based on duplex findings and preoperative CT angiograms were almost exclusively ordered by stroke physicians as part of a ‘FAST Call’ stroke protocol in radiology. When preoperative CT angiography was equivocal, duplex ultrasound performed by a vascular physiologist at the vascular centre was used to make the final decision. For this study, the index symptom was defined as the first neurological event, not the most recent event or the event that prompted the patient to seek medical attention.
The study centre has an immediate catchment area of approximately 185,000 urban adults. The vascular department is the dedicated referral centre for the three satellite hospitals within the hospital group. Established referral pathways for CEA include in-house consultations from the stroke service, who operate a weekday rapid-access TIA clinic; direct referral from a consultant physician within the hospital group; and indirect referral to the vascular laboratory for carotid duplex ultrasound. Further referrals were also received from the ophthalmology service, general practitioners and consultant physicians outside the hospital group. All procedures were performed at the vascular centre under general anaesthetic by one of three consultant vascular surgeons. The vast majority of cases were performed on one of the three-weekly scheduled vascular theatre lists, with occasional use of the shared emergency theatre facilities. All patients were initially recovered in the High Dependency Unit, as per local protocol.
Data collection and patient selection criteria
Creation of a local database for CEA was approved by the institution’s ethics committee. All patients who underwent CEA between January 2017 and December 2019 were identified from electronic theatre logs and retrospectively entered into the database. Demographic characteristics, comorbidities, smoking status and carotid imaging were documented. Defined timepoints along the patient journey from symptom onset to surgery were recorded (see Figure 1). For patients referred from external hospitals, date of outpatient appointment and/or transfer to the vascular centre were also recorded. Medical records were examined for documented causes of surgical delays.
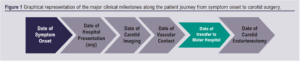
Outcomes
Outcomes were reported as per the standards published by the Society for Vascular Surgery.19 The primary outcome was the overall percentage of symptomatic CEAs performed within the recommended 14-day timeframe. Secondary outcomes included the median time from symptoms to surgery by referral site and 30-day mortality, stroke and morbidity rates.
Statistical analysis
All data was analysed using Statistical Package for the Social Sciences (SPSS) software Version 24.0 (IBM Corp, Armonk, New York, USA). Normally distributed continuous data was expressed as mean ± standard deviation (SD), while median (range) was used to describe abnormally distributed continuous data. Categorical variables were presented as count and percent. Time intervals were characterised using median (range) days. Chi-square tests and odds ratio were used to analyse categorical variables. The Mann–Whitney U test and Kruskal–Wallis test were used to analyse non-parametric data. The level of statistical significance for null hypothesis testing was p<0.05.
Results
Demographic characteristics and presenting symptoms
Demographics, risk factors, imaging and clinical symptoms are summarised in Table 1. Fifty percent (n=62) of the cohort had an acute infarct on neuroimaging. Seventy percent (n=88) had at least one presenting symptom described in the ‘Face Arm Speech Time’ (FAST) mnemonic. Twenty-eight patients (22.5%) presented with isolated ocular symptoms, while a further 14 patients had both amaurosis fugax and cerebral hemispheric symptoms.
Referral source to the tertiary vascular centre
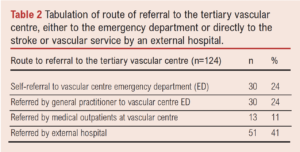
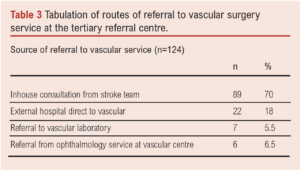
The route of referral to the vascular centre is shown in Table 2. Seventy-three patients (59%) presented directly to the vascular centre, while the remaining 51 patients (41%) initially presented to an external hospital. The source of vascular surgery referral is shown in Table 3. Of the 51 external referrals, 15 patients were transferred to the vascular centre under the stroke service, who subsequently made an in-house vascular referral. The remaining 36 external patients were referred directly to the vascular service by an outside hospital.
Symptoms to CEA: meeting the 14-day target
Time from index symptom to surgery was available for 123 patients. Fifty-four percent (n=66/123) of patients had their CEA within the 14-day target period, 78% (n=96/123) had surgery within 14 days of presentation to any hospital and 84% (n=103/123) within 14 days of review by a vascular surgeon. However, 27.6% (n=34/123) waited at least 28 days between the index event and CEA. The median interval between symptom onset and CEA was 14 days (range 2–183).
Delays between symptom onset and acute hospital presentation
The median delay between symptom onset and hospital presentation was 2 days (range 0–180). Forty-six patients (37.4%) sought medical attention on the day of the index symptom. Table 4 shows how symptom type impacted pre-hospital delays. Patients with ‘FAST’ symptoms were significantly more likely to present to an acute hospital in a timely fashion (p<0.001). There was no significant difference in time from symptoms to hospital presentation between internal and external referrals (p=0.165).
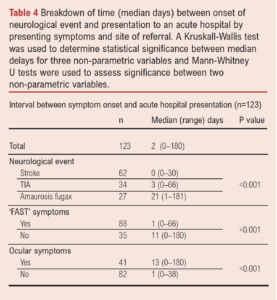
Delays between hospital admission and surgery
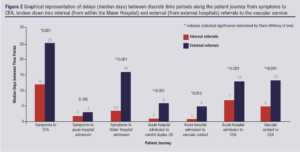
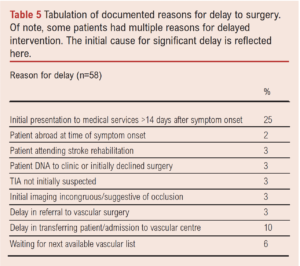
The hospital to which patients initially presented significantly impacted the timing of CEA. Almost two-thirds of patients (62.5%, n=55/88) who presented directly to the vascular centre underwent CEA within 14 days compared with 31% of patients (n=11/35) referred from external hospitals (OR 3.63, 95% CI 1.58 to 8.37, p=0.002). The median symptoms-to-surgery interval was 12 days for vascular centre presentations and 25 days for presentations to external hospitals (p=0.001). Figure 2 demonstrates how external referrals persistently encountered significantly longer delays at various discrete milestones along the journey from symptoms to surgery. The median wait between vascular centre admission to CEA was 5 days (range 0–19). The majority of CEAs were scheduled on an elective vascular list (n=115, 93.5%). Table 5 outlines the primary cause for delayed CEA across the cohort.
Perioperative complications and mortality
The 30-day stroke and mortality rates were 4.0% (n=5) and 1.6% (n=2), respectively. Both deaths were due to major perioperative strokes and occurred on the third postoperative day. Four patients (3.2%) had postoperative neck haematomas requiring re-exploration in theatre. Six patients (4.8%) had a transient nerve palsy postoperatively, including two recurrent laryngeal and four hypoglossal traction injuries.
Discussion
Best practice guidelines clearly recommend that CEA for symptomatic carotid artery stenosis be performed within 14 days of the initial ischaemic event, when the risk of further cerebrovascular accident is highest.21 Over three years in our unit, 54% (n=66/123) of symptomatic carotids met this target. Although outside the scope of this paper, general advice is that CEA within 48 hours of TIA is safe.22 In our cohort only one of 62 CEAs (1.6%) met this target; however, only eight patients presented to hospital expediently enough for TIA within 48 hours to be feasible. While this study treads on well-travelled ground by investigating delays to CEA, the specific strengths and weaknesses of the Irish healthcare system in managing this high-risk patient cohort have not been fully explored or addressed.
Pre-hospital delays
Consistent with the existing literature, the two main factors contributing to delays identified in this paper were late presentation to hospital by patients and the protracted referral process between external hospitals and the vascular centre.7,8 The speed of presentation was affected by symptom type. ‘FAST positive’ patients had a significantly shorter interval between symptom onset and hospital presentation than patients with ocular symptoms (1 vs 12 days, p<0.001).5,6,14,23,24 Similarly, the initial site of presentation was pivotal to achieving timely CEA. Direct presentation to a tertiary hospital with onsite diagnostic and surgical facilities – a ‘one-stop stroke shop’ – was significantly associated with a shorter symptoms-to-CEA interval compared with patients referred in from an outpatient setting or from satellite hospitals without a vascular capability.6,10–12,14,24–29 In our cohort, 62% (n=55/89) of patients who presented directly to the vascular centre underwent CEA within 14 days compared with 31% (n=11/35) of patients who initially presented to an external hospital (p=0.002).
External referrals encountered significant hospital-dependent delays
The median delay from symptoms to CEA was 9 days for direct presentations to the vascular centre compared with 25 days for external referrals (p=0.001). There was no significant difference in pre-hospital delays between the two cohorts (p=0.165). However, the limited access to diagnostic imaging in external hospitals has the knock-on effect of delayed identification of suitable candidates for CEA, thereby delaying vascular consultation. Patients subsequently deemed appropriate for surgical intervention had an additional wait for transfer to the vascular centre. Bed availability and local infection control policies often obstructed expeditated transfer between hospitals, reflected by a median delay of 8 days (range 0–27) between external hospital presentation and vascular centre admission. Once admitted to the vascular centre, theatre logistics accounted for 10% (n=6/58) of delays. As the vast majority of cases (92.7%, n=115) were performed on elective lists with a dedicated vascular anaesthetist, CEAs were competing against similarly urgent aortic and lower limb cases and could not always be prioritised.
‘Symptoms to surgery’ as key performance indicator for stroke management
The sources of delay identified in our study are not unique to the Irish setting. To drive sustained improvement, accurate national data on the current state of service provision and active participation from all the relevant stakeholders is necessary. To achieve these goals, the 14-day target from symptoms to CEA should be formalised as a KPI for stroke management across all Irish hospitals and a national carotid registry should be established to facilitate regular audit of all participating vascular units. These steps are essential to creating streamlined referral pathways to adequately resourced tertiary vascular units. Further lessons can be learnt from the management of hip fractures in Ireland, a comparable time-critical surgical pathology. Repair within 48 hours for all medically fit patients is an evidence-based Health Service Executive-mandated KPI, and the Irish Hip Fracture Standards are audited annually through the Irish Hip Fracture Database.30 To facilitate these meeting this target, a number of hospitals have created multi-disciplinary ‘hip fracture pathways’ with dedicated beds. A similar approach to symptomatic carotids is needed, with ring-fenced beds in receiving vascular centres and prioritised theatre access with weekly protected ‘vascular emergency’ sessions separate from elective lists.
Challenges with delayed diagnostic imaging for external referrals
As the decision to proceed with CEA at our centre was largely based on duplex ultrasound, ensuring the findings are reliable is paramount. We noted that obtaining a carotid duplex took significantly longer in external hospitals, with a median wait of 6 days between presentation and imaging. The scarcity of qualified personnel to perform specialist carotid imaging creates a critical bottleneck in the referral chain and presents a challenging resource management issue. There are not enough vascular physiologists nor is there sufficient workload to create a post in every hospital. To navigate this, one hospital within the group funds a vascular physiologist in our laboratory in exchange for expediated access; however, the workload generated by a tertiary vascular centre is too significant to extend this facility to all hospitals within the referral group. In centres without a vascular laboratory, vascular ultrasound can be performed by consultant radiologists. However, this requires the radiologist to be comfortable reporting carotid duplexes and consistent in their findings. Interestingly, having a CT angiogram made no difference in time to vascular contact or time to CEA for external referrals, as such introducing cross-sectional imaging for all cases of suspected carotid stenosis as a means of reducing delays would likely be an inefficient use of resources, as well as unnecessarily exposing patients to radiation. Potentially, a solution to reduce delays is to support radiologists and sonographers to perform carotid duplex locally though training and an initial period of performing a second quality assurance duplex at the vascular centre to corroborate the findings. It will be interesting to re-audit this target in a post-COVID landscape as the restrictions in patient movement between centres created by the pandemic have pushed hospitals to become more self-sufficient.
Limitations of study
The main limitation of this study is its retrospective nature. In particular, we noticed that the occurrence of further neurological symptoms while awaiting CEA was poorly documented. The only readily accessible objective records of subsequent embolic events for review were requests for further neurological imaging on the shared National Integrated Medical Imaging System (NIMIS). However, this likely would not capture TIAs and thus underestimates the number of patients having recurrent events. Furthermore, as the data was drawn from theatre records and not electronic consult requests, we were unable to capture patients who were potentially referred for CEA but ultimately turned down for surgery. Admittedly, these limitations weaken the impetus to drive service improvement as there is insufficient data to demonstrate that the reported delays had a negative impact on patient outcomes. Certainly, expanding a second prospective audit cycle to encompass outcomes from all patients referred to the vascular service for consideration of CEA would provide valuable additional data.
Conclusions
Just over half the patients referred to our vascular unit with symptomatic carotid disease underwent surgery within the recommended 14-day timeframe. Delays in patient presentation and delays associated with inter-hospital referrals were the two most significant factors associated with missing this target. Specific pathways between satellite hospitals and vascular centres need to be developed to expediate the treatment of symptomatic carotids, in particular expanding access to local diagnostics and protected theatre space. While the scope of this review did not include patients referred for CEA who ultimately did not undergo surgery, it highlights the need for further research in this area of stroke care provision in Ireland.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2022;1(3):77-83
Publication date:
May 5, 2022
Author Affiliations:
1 Department of Vascular Surgery, Mater Misericordiae University Hospital, Dublin 7, Ireland
Corresponding author:
Megan Power Foley
Department of Vascular Surgery, Mater Misericordiae University Hospital, Dublin 7, Ireland
Email: [email protected]











