CASE REPORT
Infection of small abdominal aortic aneurysm following a diagnosis of large vessel vasculitis and initiation of immunosuppression: presentation, diagnosis and management
Eraneva-Dibb E,1,2 Khan B,3 Davy S,4 Bera KD5
Abstract
Large vessel vasculitis is characterised by inflammation of the aorta and its major branches. This article presents the case of a patient with a 3.9 cm infrarenal abdominal aortic aneurysm at the time of diagnosis with large vessel vasculitis. Three weeks following diagnosis and initiation of immunosuppression, the patient required an emergency open repair due to confirmed methicillin-sensitive Staphylococcus aureus infection of the aneurysm. At the time of writing, there are no guidelines nor case reports in the literature to guide the management of suspected infected aneurysms in patients with active large vessel vasculitis.
Introduction
Large vessel vasculitis (LVV) typically describes two main variants – giant cell arteritis and Takayasu’s arteritis – although there is a large spectrum of LVV disease.1 Current management of LVV relies on corticosteroids, in accordance with NICE and the 2018 update of the European Alliance of Associations for Rheumatology (EULAR) guidelines, with the potential addition of long-term immunosuppression to reduce the steroid dose and achieve remission.2-4 A case of an infected abdominal aortic aneurysm (AAA) 3 weeks following a diagnosis of LVV and initiation of immunosuppression, resulting in diagnostic uncertainty and difficulty choosing the most appropriate management, is described.
Case report
A 62-year-old male, previously in good health, presented to hospital with a 1-week history of constitutional symptoms. including somnolence, drenching night sweats and one episode of melaena. The patient reported a sudden onset of symptoms as though he had been “hit by a truck”. An incidental subthreshold (3.9 cm) infrarenal AAA with significant calcium burden was noted on an investigative CT scan of the abdomen/pelvis performed during this admission (Figure 1).
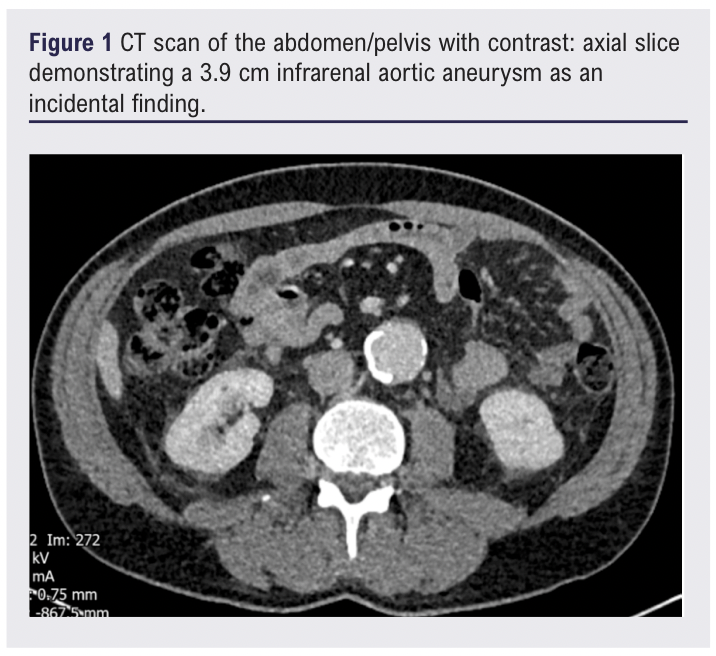
The patient’s inflammatory markers continued to rise despite initiation on co-trimoxazole for a potential urinary tract infection, suggesting a chronic inflammatory picture. A whole-body FDG-PET/CT scan 13 days after initial presentation showed mild-to-moderate FDG uptake involving the aorta and its branches, suggestive of LVV (Figures 2–4).1
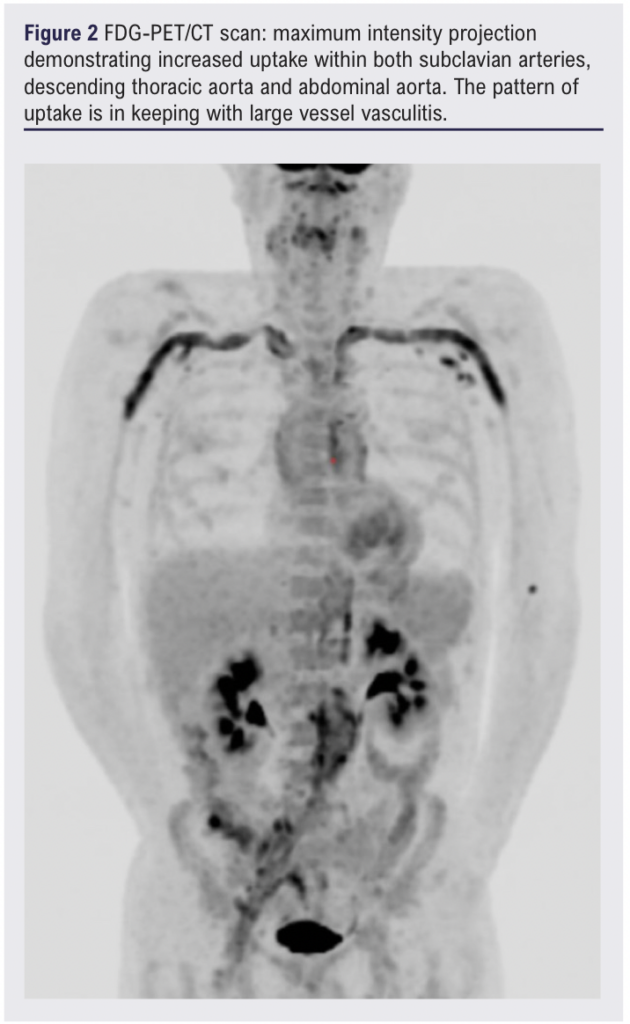
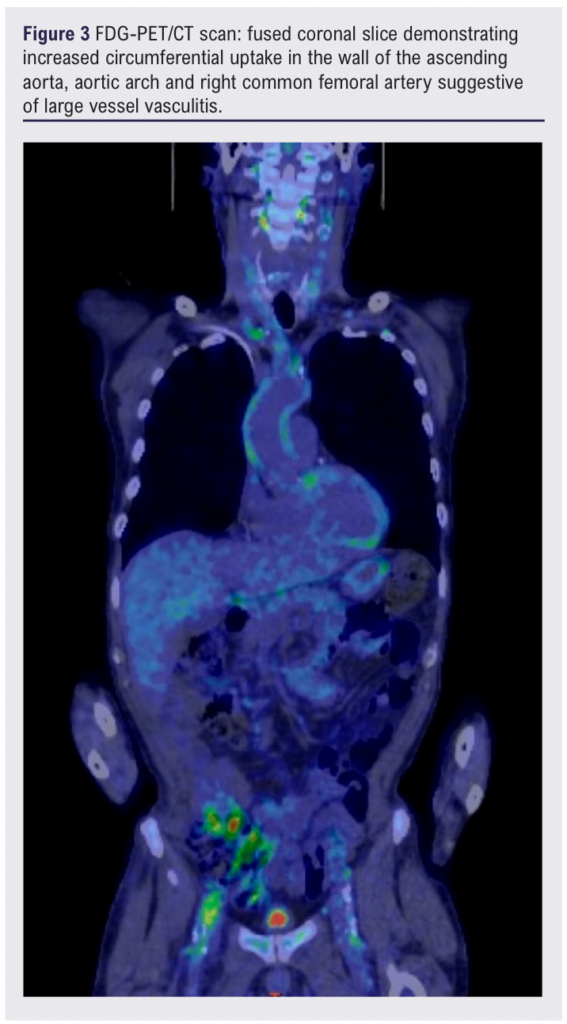
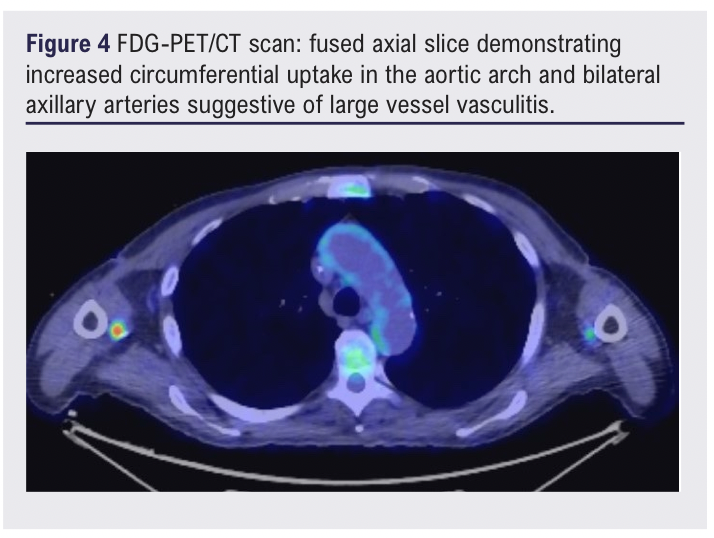
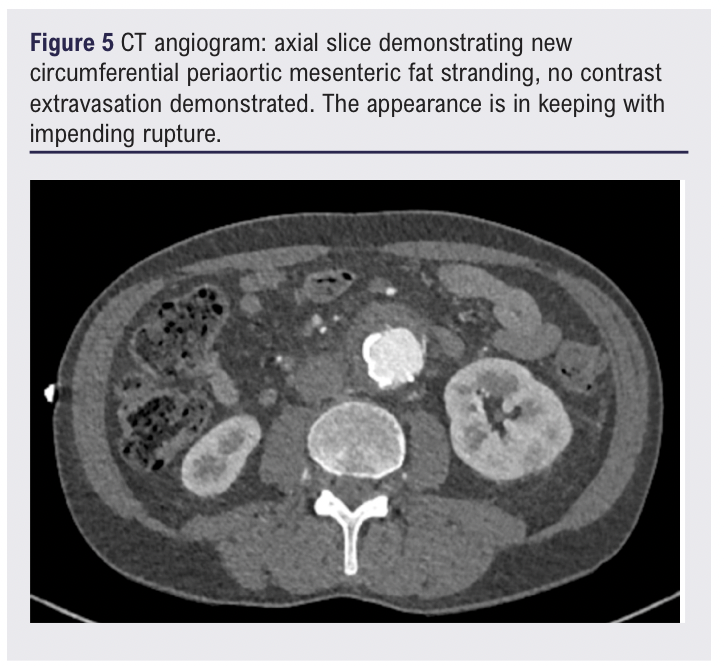
Three weeks following the diagnosis of LVV and initiation of high-dose steroids, the patient presented to the emergency department with a 3-day history of increasingly severe back pain radiating to the abdomen. A CT scan of the abdomen/pelvis showed new anterior fat stranding extending along the length of the aneurysm, concerning for impending rupture (Figure 5). Inflammatory markers were elevated on admission (white blood cell count 25.50 × 109/L, C-reactive protein 209.5 mg/L) and one of two peripheral blood cultures collected were positive for Gram-positive cocci (2/2 bottles). Splinter haemorrhages were present on both hands the following morning and a small painful cut on the left index finger was noted.
An open AAA repair using a 16 mm straight tube graft soaked in 600 mg rifampicin was performed on day 2 of the admission, with 180 mg gentamicin and 2000 mg flucloxacillin given intravenously as preoperative antibiotic prophylaxis. A silver-impregnated right aortofemoral jump graft was also inserted during this operation due to the intraoperative finding of a significantly stenosed right common iliac artery. Methicillin-sensitive Staphylococcus aureus (MSSA) was isolated from intra-aortic plaque and aortic sac specimens collected perioperatively and from the blood culture bottles positive for cocci taken on admission.
Whether postoperative management should focus on treatment of LVV or vascular infection was an area of ongoing discussion between involved specialties. The patient was initially commenced on both IV flucloxacillin and 30 mg daily oral prednisone, half the recommended dose for initial treatment of vasculitis.2-4 Considerable inflammatory soft tissue thickening involving the right aortofemoral bypass graft was seen on a CT scan 10 days following surgery, suggestive of a graft infection, and all immunosuppression was briefly withdrawn following multidisciplinary discussion. However, the patient experienced spiking fevers and rising inflammatory markers within 48 hours of this withdrawal and was recommenced on prednisone. A 2-week course of 300 mg once-daily rifampicin was added to the postoperative antibiotic regimen followed by 100 mg twice-daily doxycycline for lifelong antibiotic therapy.
Persistently active LVV was confirmed by serology and radiology 6 weeks after the surgery. It was determined that a course of six cyclophosphamide infusions would reduce the need for steroids faster than the addition of methotrexate, thereby reducing the overall infection risk.
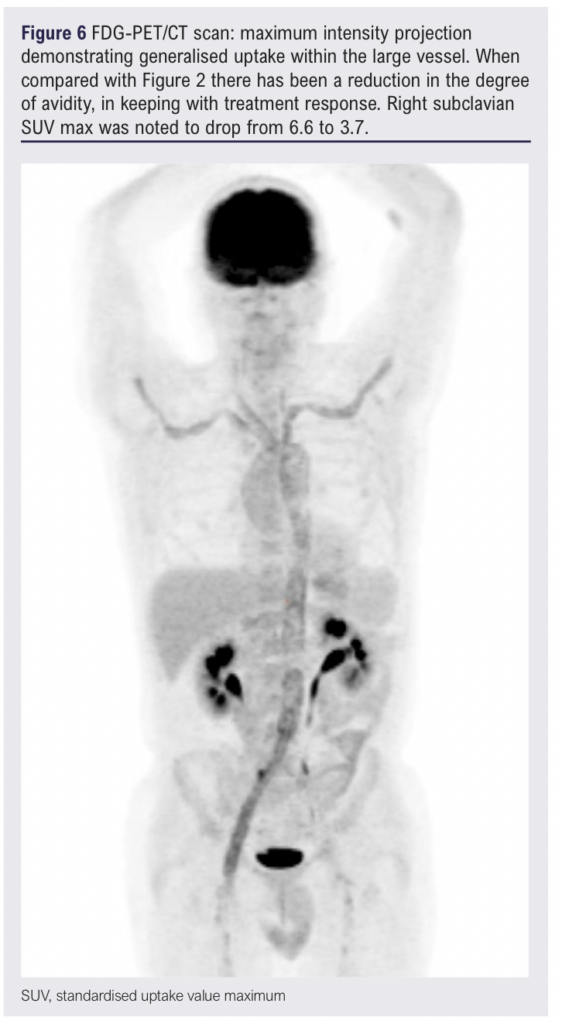
At the time of writing this case report, a partial response to six cyclophosphamide infusions has been achieved with active LVV still apparent on a FDG-PET/CT scan (Figure 6). However, the patient’s inflammatory markers are improved (erythrocyte sedimentation rate 17 mm/h, C-reactive protein 24.35 mg/L) and the patient has regained the significant amount of weight lost since first presentation.
Discussion
This case presents a complex diagnostic and management dilemma. Prior to confirmation of MSSA bacteraemia and aneurysm infection, it was uncertain whether the aneurysm changes reflected the secondary infection of a pre-existing aneurysm in the context of immunosuppression or a symptomatic aneurysm associated with active LVV disease. Once the MSSA infection was confirmed, it was reconsidered whether the initial presentation was one of a primary infective process rather than LVV; a screen for vasculitis prior to the initial FDG-PET/CT scan was negative except for mildly elevated IgA at 3.74 g/L. However, the patient’s clinical and serological response to treatment with corticosteroids and the return of symptoms on withdrawal was deemed consistent with the diagnosis of vasculitis. Taken together, a secondary infection of a pre-existing aneurysm following diagnosis of LVV is most probable.
Infected aneurysms are rare (0.7–3% of all aortic aneurysms), and of those, secondary infection of a pre-existing aneurysm represents only 3%.5 In this case, the cut finger was a potential source of bacteraemia, with secondary infection of a vulnerable region of the vasculature promoted by immunosuppression. Tailored safety netting advice given to this patient regarding potential seeding events and awareness of how a symptomatic aneurysm may present (including pyrexia and dull pain corresponding to the affected vessel) may have expedited contact with healthcare services and recognition of an infected aneurysm.5,6
Perioperative antibiotic prophylaxis was given within 60 minutes prior to surgery, in line with the Enhanced Recovery After Surgery (ERAS) Society and Society for Vascular Surgery (SVS) recommendation for open aortic surgery.7 Although there are no specific recommendations for perioperative antibiotic prophylaxis in patients with active LVV over the general population, flucloxacillin was given alongside gentamicin to provide additional Gram-positive cover, as per the advice of the local microbiology team.4,7,8 As an alternative, the 2006 systematic review cited for the ERAS/SVS recommendation suggests the addition of glycopeptide agents (vancomycin or teicoplanin) if there is a high local prevalence of antibiotic resistance or pre-existing infection, which may be appropriate if methicillin-resistant Staphylococcus aureus is suspected or isolated from intraoperative cultures.9-11 ERAS and SVS also recommend intraoperative re-dosing within two serum half-lives of the antibiotic agent(s) used, which was not required in this case.7
Both silver-coated grafts and rifampicin-soaked grafts have been used for repair of infective aneurysms. While rifampicin-soaked grafts are often used to reduce the greater risk of infection associated with synthetic grafts versus biological conduits, there is in vitro evidence to suggest that silver grafts (with or without the addition of triclosan) show superior bactericidal activity and reduced rates of bacterial resistance compared with rifampicin grafts.12 Notably, silver-coated Dacron grafts have been reported to be comparable to cryopreserved arterial homografts in terms of early and mid-term survival when used to treat abdominal aortic infection (with positive intraoperative microbiologic specimens), although two out of 11 patients who received the silver graft developed graft reinfection compared with none of 22 patients in the homograft group.13 More recently, a retrospective clinical study of 71 patients with additional risk factors for infection undergoing AAA repair, including four cases suspicious for mycotic aneurysm, reported that silver grafts had a promisingly low incidence (4.2%) of graft infection.14 Thus, silver-coated vascular grafts might offer an appropriate alternative where a biological graft is not available.
Furthermore, the potential effect of corticosteroids on aneurysms remains not well understood: multiple case studies have reported rapid expansion and spontaneous rupture of AAAs following initiation of immunosuppression, including steroids and chemotherapy.15,16 Following a paper by Lindholdt et al in 2000, which reported the mean annual expansion of AAAs to be 1.8-fold higher in COPD patients treated with oral steroids compared with those who were not, a 2017 Japanese study reported steroid use to be an independent risk factor for AAA expansion.17,18 Furthermore, a recent population-based cohort analysis investigating cardiovascular risk with steroid use noted a hazard ratio of 1.84 (95% CI 0.30 to 11.32) for the occurrence of AAA in patients diagnosed with vasculitis taking steroid doses equivalent to <5 mg daily oral prednisone, increasing to 3.47 (95% CI 0.79 to 15.22) for doses equivalent to 15–24.9 mg daily prednisone.19 It should be noted that no association between immunosuppressant drugs prescribed for non-transplant pathology was found in a subsequent paper published in 2022.20
Conclusion
At the time of writing, there are no guidelines for the management of patients with both active LVV and an infected aneurysm. This case highlights potential risks associated with high-dose steroid therapy, arguing for prompt recognition and treatment of potential infection in these patients. Management of symptomatic aneurysms in immunosuppressed patients should carry a high index of suspicion for an infected aneurysm, for which use of either a biological or silver-coated vascular graft may be most appropriate. Careful monitoring of both disease processes allowed tailoring of treatment to achieve a favourable outcome in this case.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2025; Online ahead of publication
Publication date:
July 3, 2025
Author Affiliations:
1. Medical Sciences Division, University of Oxford, Oxford, UK
2. Magdalen College, University of Oxford, Oxford, UK
3. Department of Radiology, John Radcliffe Hospital, Oxford, UK
4. Department of Neuroradiology, John Radcliffe Hospital, Oxford, UK
5. Department of Vascular Surgery, John Radcliffe Hospital, Oxford, UK
Corresponding author:
Kasia D Bera
Department of Vascular Surgery, John Radcliffe Hospital, Headley Way, Headington,
Oxford OX3 9DU, UK
Email: [email protected]











