CASE REPORT
Axillary EndoVac procedure: a novel hybrid procedure for an infected axillary-profunda bypass
Forsyth JM,1 McPherson S2
Abstract
This paper reports an axillary artery EndoVac procedure for the management of an infected left axillary-profunda polytetrafluoroethylene (PTFE) bypass. Our patient presented with peri-graft infection and dehiscence of the distal anastomosis. Three separate surgical procedures subsequently took place over a seven-day period: day 1 required profunda artery ligation with sub-total explantation of the mid to distal PTFE bypass, day 2 required a left above-knee amputation, and the final operation on day 7 was the left axillary artery EndoVac procedure.
The EndoVac procedure involved endovascular relining of the axillary artery with a covered stent graft, immediately followed by explantation of the remaining proximal PTFE graft and vacuum-assisted closure (VAC) application within the same operative setting. This resulted in successful wound healing within six weeks. It is not recommended currently as first-line management in treating vascular graft and endograft infections of the axillary vessels but the EndoVac approach should be considered in a select cohort of patients.
Introduction
Vascular graft and endograft infections are challenging to manage. They can present insidiously or acutely with sepsis and bleeding. The gold standard approach is complete excision of all infected prosthetic material. Other approaches include covered stent placement with long-term suppressive antibiotics, conservative management with antibiotics alone, or a purely palliative approach. Major challenges in this patient cohort include significant co-morbidities, co-existent sepsis and bleeding. Compromised autologous vein availability may also be an issue. This case report highlights the use of the axillary EndoVac procedure as an innovative management strategy for an infected axillary-profunda polytetrafluoroethylene (PTFE) graft.
Case report
A 78-year-old man was referred with left chronic limb-threatening ischaemia (left foot rest pain but no tissue loss). His past medical history included kissing iliac stents, left common femoral artery (CFA) endarterectomy, hypertension, type 2 diabetes (T2DM), transient ischaemic attack, chronic obstructive pulmonary disease (COPD) and epilepsy. Computed tomography angiography (CTA) revealed a moderately diseased infra-renal aorta, patent but diseased right iliac system with a patent right iliac stent, severely diseased right common femoral artery, left common and external iliac full-length occlusion with collateral vessels filling a patent left CFA endartectomy site, a patent profunda artery, and a long superficial femoral artery (SFA) occlusion with distal crural vessel disease. Multidisciplinary (MDT) consensus supported a left axillary-profunda bypass, which was performed with otherwise rigorous infection control measures and sartorius flap coverage of the distal anastomosis.
The patient had a type 2 myocardial infarction peri-operatively that was managed medically. He was discharged home on day 9. At this time he was systemically well and apyrexial, with no wound site concerns and with a warm pink left foot with resolved rest pain. His C-reactive protein (CRP) on discharge was 34 mg/dL and down-trending. Four days after discharge, he presented again with profound haemodynamic collapse, with a swollen erythematous left groin with fresh blood seeping from the groin surgical site. There was erythema and tenderness along the course of his lower graft tunnelling site. His inflammatory markers were now significantly raised with a CRP of 182 mg/dL and a white blood count (WBC) of 29×109/L. He was also complaining of chest pain and his ECG showed ST segment depression. CTA revealed haematoma in the left groin containing gas, with contrast extravasation from the distal graft – profunda artery anastomosis (Figure 1), and fluid around the lower portion of the graft.
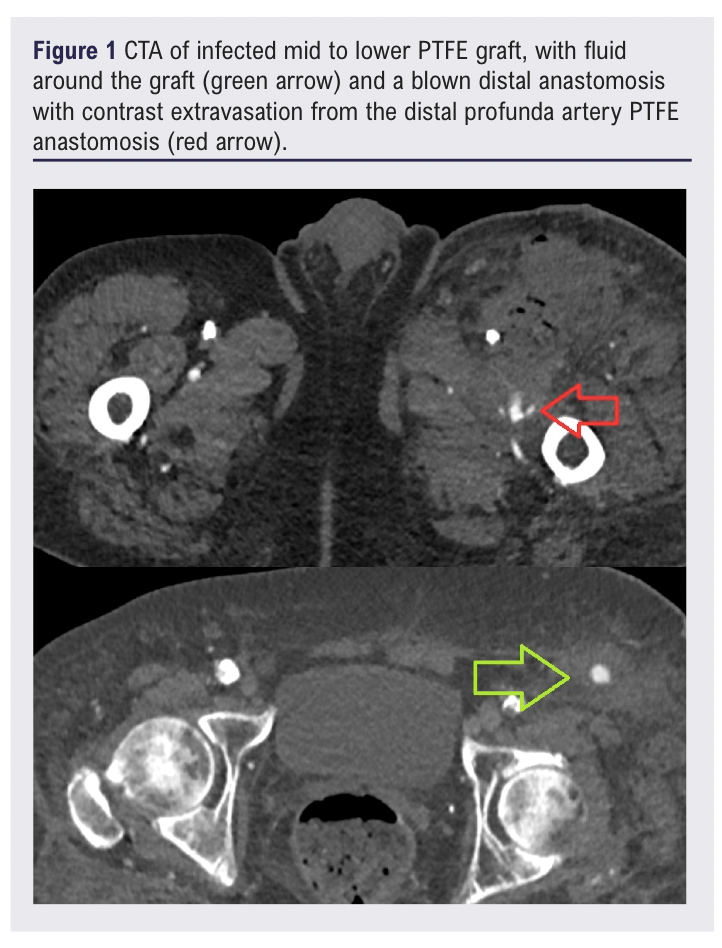
The patient was taken to theatre for ligation of the profunda artery and explantation of the PTFE graft. The patient’s systolic blood pressure was 60 mmHg at the start of the operation, with very high vasoconstrictor requirements throughout. The PTFE graft was encircled by pus up towards mid-abdomen but above this point another incision did not reveal gross infection around the graft at the level of the nipple. Within a damage control context a sub-total graft explantation was performed with around 5 cm of graft being left in situ above the nipple. The patient went back to the intensive care unit (ICU) for maximal medical support.
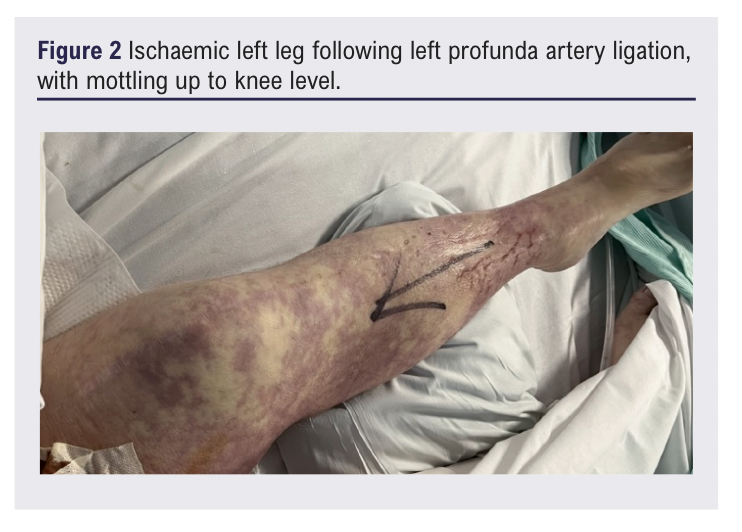
The following morning his left leg was profoundly ischaemic (Figure 2), necessitating a high left above-knee amputation. The patient slowly recovered, and the explanted graft grew methicillin-sensitive Staphylococcus aureus (MSSA), sensitive to flucloxacillin.
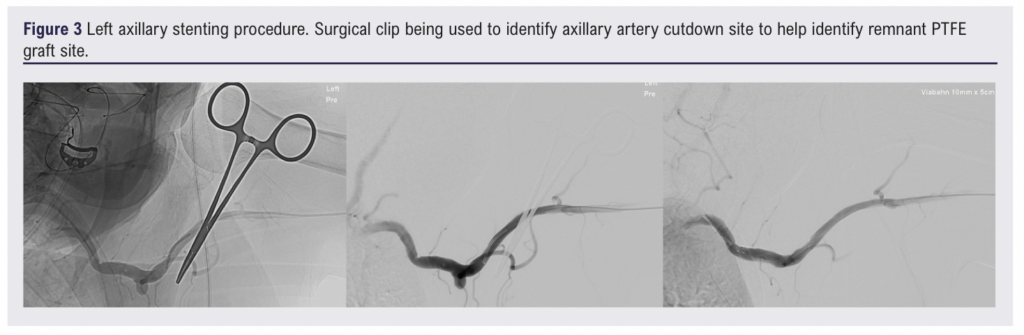
There were concerns that the remaining proximal PTFE graft was highly likely to be infected and about future haemorrhage from the axillary-PTFE anastomosis. Therefore on day 7, under general anaesthetic, via a left brachial artery cutdown, a 11 mm x 5 cm Viabahn self-expanding stent graft (W L Gore & Associates Inc, Flagstaff, Arizona, USA) was placed in the left axillary artery, centred on the surgical-graft anastomosis, with immediate occlusion of the residual graft (Figure 3). The remaining PTFE graft was explanted, and following 20 minutes of direct observation to ensure haemostasis a vacuum-assisted closure (VAC) device was applied. A white polyurethane foam sponge was placed directly over the axillary vessel and a black polyurethane foam sponge was used on top to bring the VAC material up to the skin (Figure 4).
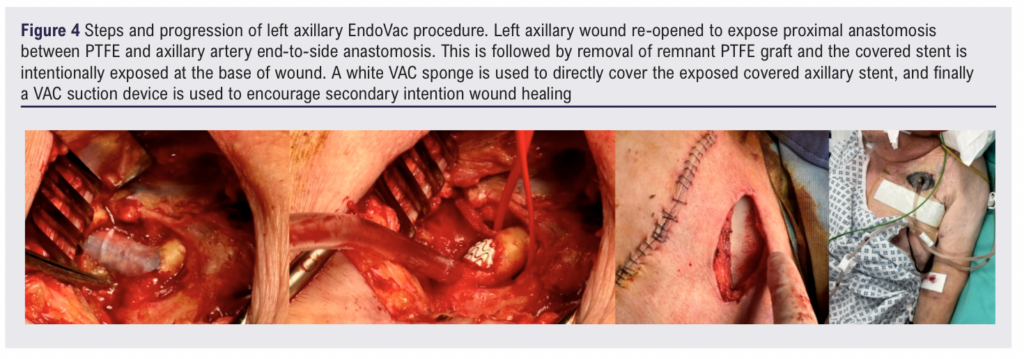
For the first 24 hours the VAC was applied at a continuous pressure of 125 mmHg. The following day and beyond the VAC was set intermittently at 125 mmHg for four hours followed by a one-hour rest, with the cycle then repeating. The axillary vessels were covered within a few days and then the VAC was switched to black polyurethane only until the wound was healed enough to allow a switch to regular dressings and normal wound packing.
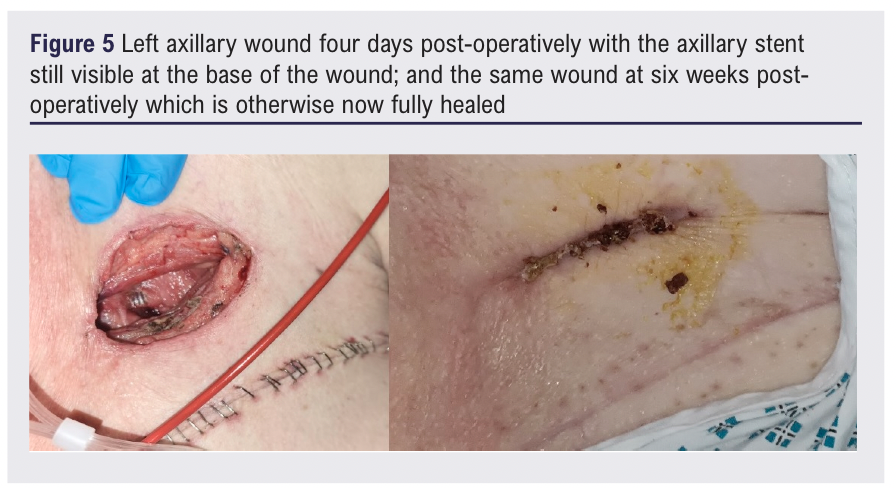
The left axillary and brachial artery wounds healed over six weeks (Figure 5) and at two months post-operatively the patient was continuing to recover in an intermediate care unit. The patient was continued post-operatively long-term on aspirin 75 mg OD and rivaroxaban 2.5 mg BD. There was no bacterial growth from the residual proximal remnant PTFE graft anastomosed to the axillary artery on microbiological culture but long-term oral antibiotic therapy was continued as treatment for an intra-vascular infection.
Discussion
The reported experience with early axillary graft infections presenting with sepsis and haemorrhage is limited. In such scenarios, initial life-saving damage limitation (haemorrhage control and safe removal of infected material) surgery would seem a sensible option. The most frequent bacteria isolated in such scenarios appear to be Staphylococcus aureus.1-3 There is variation in subsequent strategies, which include antibiotics alone without any surgical treatment, full or partial explantation of the infected graft, and differing approaches to revascularisation. No one strategy emerges as clearly superior, and amputation rates (18-57%) and mortality rates (16-22%) are significant.1-5
Given this context and our previous successful use of the EndoVac procedure in a high-risk surgical patient with a carotid Dacron patch infection, it seemed reasonable to consider the EndoVac procedure in this patient whom we deemed to be at high risk of future catastrophic axillary artery haemorrhage.6 This was supported by the European Society for Vascular Surgery clinical practice guidelines.7 However, unlike our reported carotid EndoVac case where stent-graft insertion was performed a few days before the EndoVac operation, on this occasion the axillary stent-graft and EndoVac procedure were performed as a single procedure in a hybrid theatre.
This case report presents the relatively short-term positive outcome for this patient, but the long-term results of a small EndoVac series appear relatively reassuring. Shebab et al report satisfactory long-term results for nine patients after carotid EndoVac procedures who were followed up for a median of 7.6 years.8 All patients healed with no graft-related re-infections; however, in-stent stenoses or occlusions occurred in three patients despite dual antiplatelet therapy. Thus, consideration should be given to anticoagulation and surveillance.9
Conclusion
Infected axillo-femoral bypasses are rare but extremely challenging events with high morbidity and mortality rates. Many of these infections will present in frail, co-morbid, high-risk patients with haemodynamic instability due to sepsis and/or bleeding. There are many different surgical approaches, and conservative and palliative approaches may be relevant. This case report describes an additional approach worth considering in the therapeutic armamentarium. Following initial damage control sub-total graft explantation, the axillary EndoVac procedure can be performed either as a single-stage hybrid procedure or as a two-stage (endovascular stent-graft first, surgical graft explantation/ VAC application second) procedure. The single-stage approach used here suggests stent grafting and EndoVac as an emergency option.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2025;4(2):111-114
Publication date:
February 27, 2025
Author Affiliations:
1. Consultant Vascular Surgeon, Leeds Vascular Institute, Leeds General Infirmary, Leeds, UK
2. Consultant Vascular Radiologist, Leeds Institute of Radiology, Leeds General Infirmary, Leeds UK
Corresponding author:
James Michael Forsyth
Consultant Vascular Surgeon, Leeds Vascular Institute, Leeds General Infirmary,
Leeds, LS1 3EX, UK
Email: [email protected]











