ORIGINAL RESEARCH
Cardiovascular morbidity, mortality and risk management in patients with abdominal aortic aneurysms
Kwan JY,1,2 Dar T,1,2 Davies H,1,2 Stocco F,1,2 Scott DJA,1 Bailey MA,2 Coughlin PA1
Plain English Summary
Why we undertook the work: Abdominal aortic aneurysm (AAA) is a condition in which the main blood vessel in the abdomen dilates. Most people who develop an AAA do not die of the aneurysm but from other vascular complications such as heart attacks and strokes (major adverse cardiovascular event (MACE)). To help reduce this risk, guidelines suggest that patients are prescribed medications to lower cholesterol levels (lipid-lowering therapy) and to prevent blood clots from forming (antiplatelets). This review aims to measure how likely patients with an AAA are to experience or die from a MACE.
What we did: A review was carried out to consolidate studies that had previously looked at the chances of people with an AAA developing or dying from a MACE. Details about the patient, their health conditions and the medications they were receiving were gathered.
What we found: Out of the total group of 78,500 patients studied, 14.5% of people died from a cardiovascular event over a 5-year period. Only approximately 60% of patients were prescribed lipid-lowering therapy and antiplatelet therapy. On average, each year, 5.43% of the group was at risk of dying from a cardiovascular event.
What this means: This review shows that many patients with AAA are not getting the best possible treatments. There is still a high rate of MACE-related health problems and deaths in these patients. People diagnosed with an AAA usually have just one appointment with a vascular surgeon specifically for this condition. After that, we suggest that their regular primary care doctor handles ongoing cardiovascular-related check-ups and treatment as part of their regular care. More focus should be placed on improving the use of lipid-lowering therapy and antiplatelet therapy within primary care to help improve their outcomes.
Abstract
Introduction: Cardiovascular events are the most common cause of mortality in patients with an abdominal aortic aneurysm (AAA) regardless of intervention to treat the aneurysm. This systematic review aims to quantify the risk of cardiovascular morbidity and mortality in all patients with AAA before and after repair.
Methods: The review was conducted in line with the framework of Cochrane reviews and Standard Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Electronic databases were searched for studies that published cardiovascular mortality and/or morbidity rates of people diagnosed with an AAA. Studies only discussing AAA-related deaths or papers looking at aneurysms caused by connective tissue disorders, thoracic aortic aneurysms or ectatic abdominal aortas were excluded. Data on patient demographics, prevalence of comorbidities and medical therapy were extracted where possible.
Results: 17 studies with 78,500 patients were included. The weighted mean prevalence of antiplatelet therapy and lipid-lowering therapy prescriptions was 62.8% and 60.2%, respectively. Pooled mortality rates secondary to myocardial infarction were 5.7% at 5 years with a 2.87% risk of death per year and 11.4% at 10 years with a 1.48% risk of death per year. The pooled mortality rate secondary to an acute cerebrovascular event was 1.9% at 5 years with a 0.38% risk of death per year. The pooled overall cardiovascular mortality rate was 14.5% at 5 years with a 5.43% risk of death per year.
Conclusions: This review demonstrates suboptimal prescription of best medical therapy alongside a significant incidence of cardiovascular morbidity and mortality in patients with AAAs. Greater emphasis should be placed on optimisation of antiplatelet therapy and lipid-lowering therapy in primary care and vascular surgery services with the aim of improving cardiovascular-related outcomes for this patient cohort.
Introduction
An abdominal aortic aneurysm (AAA) is a focal dilation of the abdominal aorta with a diameter of 3.0 cm or more. Within the UK the national screening programme has shown an AAA prevalence in men aged >65 years of 1.57%, similar to that in the USA.1
The implementation of screening programmes for AAAs across the UK and other countries has resulted in an approximately 50% reduction in aneurysm-related mortality.2 However, it is recognised that the previously documented historical rupture rates may be higher than those observed in practice today. For example, data from the UK NAAASP suggest rupture rates of around 0.4% per annum for a large 5.0–5.5 cm AAA and around 0.03% per annum for a small aneurysm of 3 cm.3 Rupture is therefore unlikely to be the primary cause of death in this cohort of patients.
The Multicentre Aneurysm Screening Study (MASS) trial demonstrated that cardiovascular events are the most common cause of mortality in men with AAA, regardless of intervention to treat the aneurysm.4,5 The European Society for Vascular Society (ESVS) 2019 and 2024 AAA guidelines therefore recommend consideration of antiplatelet therapy, lipid-lowering therapy and antihypertensives in all AAA patients to reduce the incidence of major adverse cardiovascular events (MACEs; defined as incidence of non-fatal acute myocardial infarction, non-fatal stroke and cardiovascular death).6,7 Yet such practice is still not commonplace.
Existing work studying the association between AAA and cardiovascular risk has been mainly focused on small aneurysms. Data from the UK Small Aneurysm Trial suggested that, for every 8 mm increase in aneurysm diameter, the relative risk of cardiovascular death increased by 1.34.8 In addition, Bath et al concluded that patients with a small AAA have an annual risk of cardiovascular death of 3.0% (95% CI 1.7% to 4.3%), a similar risk to those patients who have already experienced a MACE.2,9
The aim of this systematic review is to quantify the risk of cardiovascular morbidity and mortality in all patients with AAA before and after repair, consolidating the evidence regarding the use and degree of medical management of cardiovascular risk in this patient cohort.
Methods
This review was registered on Prospero, carried out within the framework of Cochrane reviews and reported in line with the Standard Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A literature search of Cochrane, Medline and Embase databases was performed through OVID with no limitations on date of publication or by language. The search strategy used (((abdominal aortic aneurysm* or AAA) and (heart failure or acute coronary syndrome or myocardial infarct* or heart attack or angina or coronary disease or myocardial ischaemia or ischaemic attack or stroke or brain infarct* or cerebral infarct* or cerebrovascular or mortalit* or cardiovascular or cardiovascular disease*)).ti.) was adapted from that of a previous systematic review analysing cardiovascular disease and death in patients with small AAAs.1 An additional retrocursive search was conducted through the bibliographies of included studies and the reference lists of relevant systematic reviews.
Type of studies
This review included all prospective observational studies and randomised controlled trials published as full papers reporting cardiovascular mortality and/or morbidity rates of people diagnosed with an abdominal aortic aneurysm. Patients undergoing all forms of treatment (surveillance, endovascular aneurysm repair/open repair for elective/ruptured aneurysms) including those deemed unfit for treatment were all included. We specified a minimum study number of 50 participants and a minimum of 1-year follow-up for mortality, with no upper limit of follow-up.
Studies not published in English and those that only discussed AAA-related deaths secondary to AAA rupture were excluded. Papers evaluating the outcomes of a non-operative intervention on AAA patients (eg, looking at the effect of red blood cell transfusion in AAA patients) were also excluded. Papers looking at AAA patients aged <45 years were excluded to account for patients with connective tissue diseases, which have a different aetiology from degenerative AAA. Papers looking at thoracic aortic aneurysms or patients with ectatic abdominal aortas (defined as a maximal diameter of 2.5–2.9 cm) were also excluded.
Type of outcome measures
The primary objective of this review was to quantify the risk of cardiovascular morbidity and mortality in patients with an AAA. Outcomes were guided by the traditional three-point MACE outcome, defined as the incidence of non-fatal acute myocardial infarction, non-fatal stroke and cardiovascular death.10 Therefore, cardiovascular morbidity was defined as the incidence of a new non-fatal MACE following diagnosis of AAA, and cardiovascular death/mortality was defined as any non-aneurysm rupture-related death caused by a MACE. Mortality was stratified by follow-up intervals of 1, 5 and 10 years. Cardiovascular mortality occurring <30 days after an invasive treatment was considered post-procedural and therefore excluded. The secondary objective of this review was to quantify the prevalence of optimal medical therapy, defined as antiplatelet therapy and lipid-lowering therapy.
In addition to the outcome described above, data on patient demographics, prevalence of comorbidities (diabetes, hypertension, chronic kidney disease, peripheral arterial disease, ischaemic heart disease, previous cerebrovascular incident) and treatment strategy (medical treatment, endovascular repair, open repair) were collected.
Assessment of risk of bias
Two authors independently assessed the quality and validity of the included papers using the Critical Appraisal Skill Programme (CASP) checklist for cohort studies and randomised controlled trials.
Statistical analysis
MetaXL version 5.3. statistical software was used to assess study heterogeneity and to calculate the rates of each outcome using a meta-analysis of proportions. Owing to significant heterogeneity, a random-effects model with double arcsine transformation was used. Survival function: was used to approximate the risk of death per year assuming a constant hazard of death. A subsequent sensitivity analysis was conducted, excluding studies with a publication rate prior to 1 January 2000, so that only studies that reflect contemporary cardiopreventive medications and guidelines were included.
Results
Study and baseline characteristics
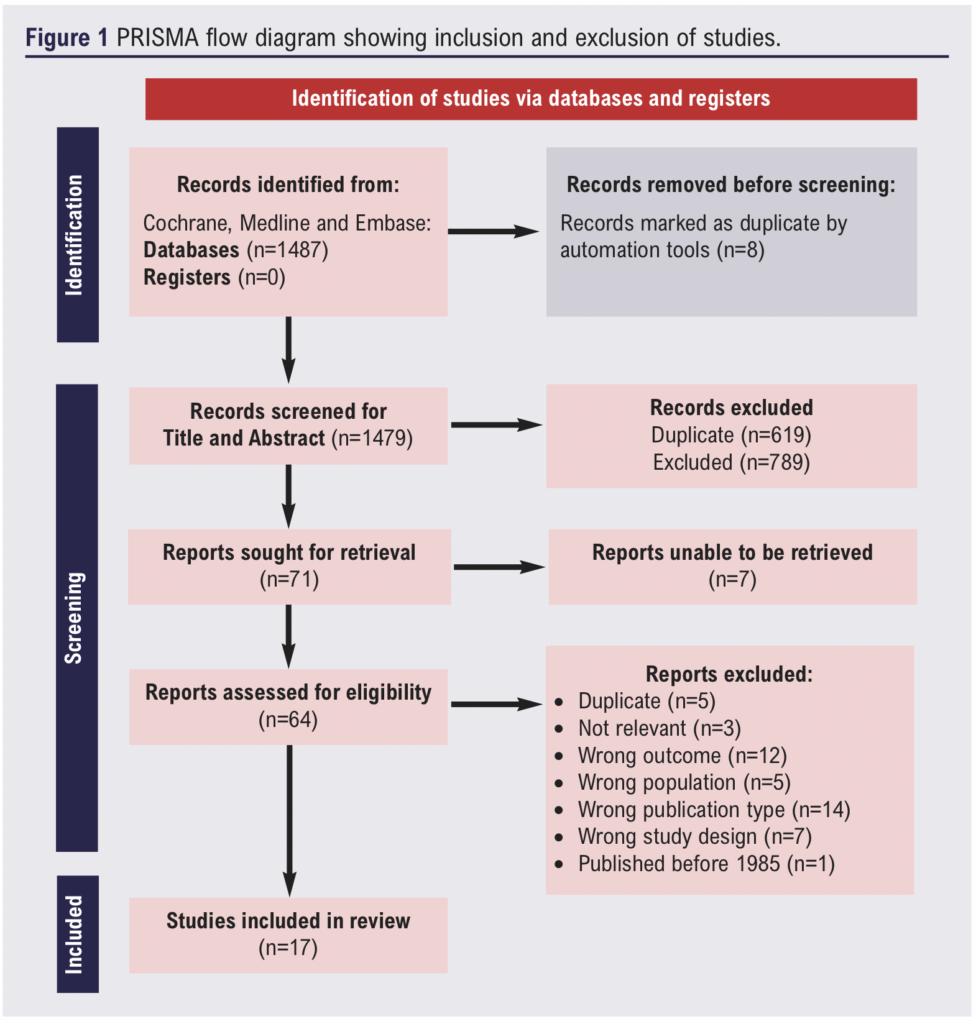
The literature search identified 1487 potential articles which was refined to 17 studies following assessment.9,11-26 The PRISMA flow diagram is illustrated in Figure 1. Qualitative assessment of these papers suggested that the overall quality of the available evidence was low. Notably, there was significant variability in definitions of outcomes and length of follow-up within the series.
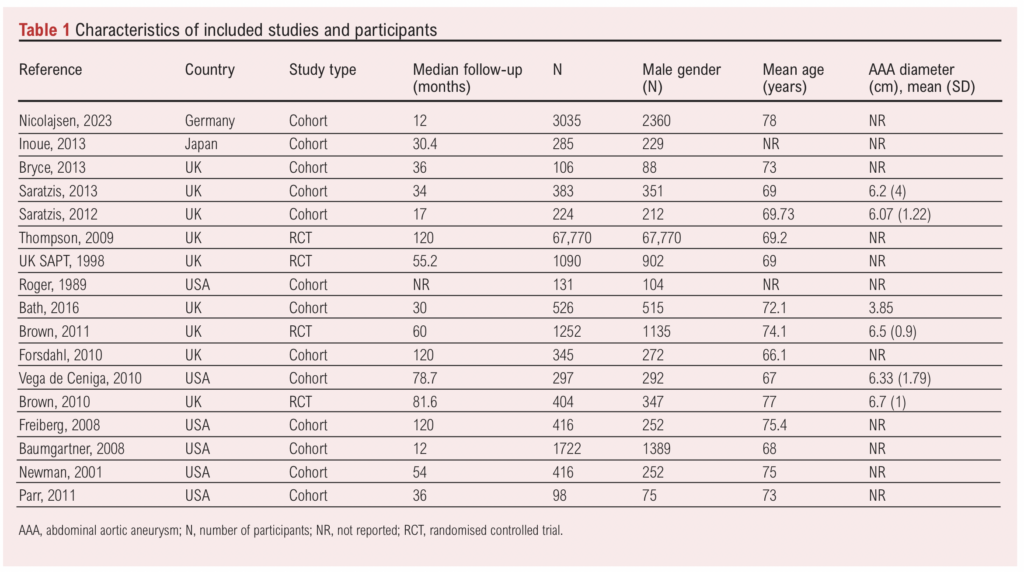
Of the 17 studies, four were randomised controlled trials and 13 were cohort studies. Publication dates ranged from 1989 to 2023. Study size ranged from 98 to 67,770 patients and mean follow-up was 56 months. A total of 78,500 patients were included, of which 97.5% (n=76,545) were men, and the mean age was 71.7 years. Characteristics of the included studies and participants are shown in Table 1.
Prevalence of cardiovascular comorbidities
Overall, 15 studies reported the prevalence of diabetes, hypertension, chronic kidney disease, peripheral arterial disease, ischaemic heart disease and previous cerebrovascular events in the baseline characteristics of the study (Table 2).

A total of 13 papers consisting of 9,757 patients showed a weighted mean prevalence of 16.0% for diabetes mellitus; 15 papers consisting of 10,326 patients showed a weighted mean prevalence of 57.9% for hypertension; four papers consisting of 3,723 patients showed a weighted mean prevalence of 19.5% for chronic kidney disease; five papers consisting of 4,037 patients showed a weighted mean prevalence of 35.2% for peripheral arterial disease; five papers consisting of 1,251 patients showed a weighted mean prevalence of 27.2% for ischaemic heart disease; and eight papers consisting of 4,877 patients showed a weighted mean prevalence of 17.4% for previous cerebrovascular event.
Prevalence of optimal medical therapy
The prevalence of antiplatelet therapy and lipid-lowering therapy was assessed in 10 papers (Table 2). Eight papers consisting of 8,233 patients showed a weighted mean prevalence of 62.8% (27.4–90.8%) for patients prescribed antiplatelet therapy and nine papers consisting of 7,750 patients showed a weighted mean prevalence of 60.2% (35.1–77.3%) for patients prescribed lipid-lowering therapy. None of the papers reported the prevalence of concurrent use of antiplatelet therapy and lipid-lowering therapy. Two papers consisting of 4,757 patients showed a weighted mean prevalence of 84.6% for patients prescribed antihypertensive therapy. Four other papers reported the use of agents such as beta blockers and diuretics. However, it was not specified whether these agents were initiated for hypertension or cardiac indications so they have not been included in the analysis.
Cardiovascular morbidity
Three studies reported the incidence of non-fatal acute myocardial infarction at 1 year. The smallest study reported a prevalence of 6.3% amongst the 526 patients included, whilst the largest study containing 3,035 patients showed an incidence of 4.4%. The weighted event rate was 3.6% for a total of 5,283 patients. Two studies reported the incidence of non-fatal acute myocardial infarction at 5 years, demonstrating a weighted event rate of 4.1% for a total of 1,656 patients.
Two studies reported the incidence of non-fatal acute cerebrovascular event at 1 year. The weighted event rate was 3.7% for a total of 4,757 patients. Two studies reported the incidence of non-fatal acute cerebrovascular event at 5 years, demonstrating a weighted event rate of 4.3% for a total of 1,656 patients.
Cardiovascular mortality
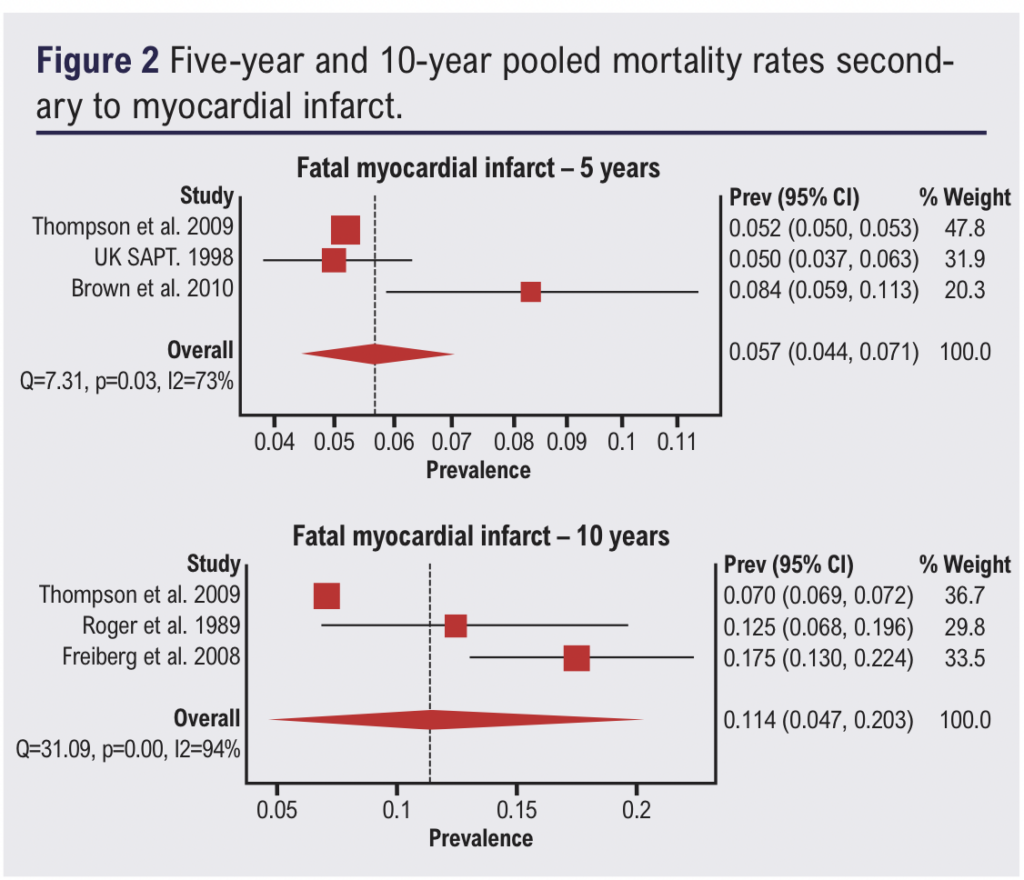
Three papers reported rates of cardiovascular mortality secondary to acute myocardial infarction at 5 years and three papers at 10 years. Pooled mortality rates secondary to myocardial infarction were 5.7% (4.4–7.1%) at 5 years with a 2.87% risk of death per year and 11.4% (4.7–20.3%) at 10 years with a 1.48% risk of death per year (Figure 2).
Five papers reported rates of 5-year cardiovascular mortality secondary to an acute cerebrovascular event; the pooled mortality rate was 1.9% (1.0–3.1%) at 5 years with a 0.38% risk of death per year (Figure 3).

Four papers reported rates of total undefined cardiovascular mortality at 5 years; the pooled mortality rate was 14.5% (7.6–23.0%) with a 5.43% risk of death per year (Figure 4).

All-cause mortality
Five papers reported overall mortality rates at 5 years and five papers at 10 years. The pooled overall mortality rate was 28.1% (19.5–37.7%) at 5 years with a 7.05% risk of death per year and 38.3% (30.9–46.1%) at 10 years with a 4.86% risk of death per year (Figure 5).

Sensitivity analysis
To strengthen the robustness of our findings, we performed a sensitivity analysis focusing exclusively on studies that reflect contemporary cardiopreventive medications and guidelines (ie, studies published before the year 2000 were excluded). Overall 5-year rates of cardiovascular mortality, cardiovascular mortality specifically from acute myocardial infarction and cardiovascular mortality specifically from stroke were 13.3% (5.5–23.4%), 6.3% (3.5–9.9%) and 1.3% (1.0–1.6%), respectively, which were similar to the primary analysis results of 14.5%, 5.7% and 1.9%.
Discussion
Using 5-year mortality rates, this review suggests the estimated per-year incidence of overall cardiovascular death, cardiovascular death specifically from acute myocardial infarction and cardiovascular death specifically from stroke are 5.43%, 2.87% and 0.38%, respectively. The mean incidence of cardiovascular mortality in the general global population is estimated to be 0.2% per year, which suggests that a patient diagnosed with an AAA will experience an approximately 27 times greater risk of cardiovascular death compared with the general population.27 The high rates of cardiovascular-related mortality demonstrated in this review are in keeping with previous studies looking at AAA subgroups. Bath et al (2015) quoted a 3% risk per year of cardiovascular death in patients with a small AAA,2 while Sharma et al (2023) quoted a 2.31% incidence of cardiovascular death in patients with an unrepaired AAA.28
Increasing awareness of the substantial cardiovascular risk faced by patients with an AAA is reflected in the latest published ESVS 2024 guidelines, which has stated as a Class I recommendation that “all patients with abdominal aortic aneurysm should receive cardiovascular risk factor management with smoking cessation, blood pressure control, statin and antiplatelet therapy and lifestyle advice”. Our review demonstrated weighted mean prevalence rates of 60.2% for lipid-lowering therapy, which is lower than a UK-based study showing statin prescription rates of 81% for patients with cerebrovascular disease and 75% for peripheral arterial disease.29 These numbers suggest that there is room to improve the uptake of long-term cardioprotective medical therapy.
Research and funding should be directed towards interventions targeted at promoting lifestyle changes and optimising prescription rates of risk factor-lowering medications within this patient cohort.30 Primary care and vascular surgery services are suitable settings to achieve this. However, patients diagnosed with AAA are typically seen by a vascular surgeon for a single consultative visit regarding this condition. Consequently, whilst specific cardiopreventive medications can be initiated in secondary/tertiary care, the majority of ongoing cardiovascular risk management should be conducted within primary care, where continuous monitoring and management of comorbidities can be effectively integrated into routine care. Factors such as time constraints, lack of appropriately trained staff and level of clinician education and confidence serve as barriers.31 One effective approach was reported by Smits et al in 2023 in the Netherlands.32 This involved a dedicated practice nurse protocol and annual clinician education meetings as part of an integrated cardiovascular risk management program.
The main limitation of this study is the significant variation in population and defined outcomes between included papers; considerable heterogeneity was reflected in the calculated χ2 score. A wide range of definitions was used for cardiovascular mortality and the cardiovascular diseases identified, yet the results still reflect adverse cardiovascular events. Other factors contributing to heterogeneity included variations in follow-up timepoints and follow-up length, and different prevalences of cardiovascular risk factors. Given the heterogeneous nature of the populations, exact treatment strategies (conservative, endovascular repair, open surgical repair) and numbers of patients deemed unfit for intervention were difficult to determine. The small number of studies identified meant that it was not feasible to perform a sensitivity analysis or subgroup analysis due to the likelihood of generating false positive and false negative results.
The papers included in this study span from 1989 to 2023. Roger et al (1989) reported the highest rate of fatal cerebrovascular events and it is also the oldest paper included in this study.18 There has been a decline in stroke rates over time accompanied by improvements in pharmacotherapy for cardiovascular risk reduction.33 Therefore, the older papers in this study may not accurately reflect current event rates. However, consistency between the results of primary analysis and the results of sensitivity analysis strengthen the conclusions and credibility of the findings.
It is notable that women were poorly represented in the included papers. The risk factors, indications for treatment and outcomes following repair of AAAs in women is less well understood than in men.34 Ninety-seven percent of the patients in this study were men, which limits the generalisability of the findings to women with AAAs.
Conclusion
This review demonstrates a significant incidence of comorbidities and eventual MACE in patients with AAAs of all sizes, alongside suboptimal prescription of best medical therapy. Greater emphasis should be placed on optimisation of antiplatelet therapy and lipid-lowering therapy in this patient cohort with the aim of improving cardiovascular-related outcomes. Vascular surgery services provide opportunities to initiate cardiopreventive medical therapy and primary care services as a platform for ongoing cardiovascular risk management, where continuous monitoring and management of comorbidities can be effectively integrated into routine care.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;4(1):18-24
Publication date:
November 19, 2024
Author Affiliations:
1. Leeds Vascular Institute, Leeds Teaching Hospitals NHS Trust, UK
2. Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, UK
Corresponding author:
Jing Yi Kwan
Leeds Vascular Institute, Leeds General Infirmary, Great George Street, Leeds LS1 3EX, UK
Email: [email protected].











