TRIAL PROTOCOL
Community WALKing and home-baSed circuiT tRaining in peOple liviNG with intermittent claudication (WALK-STRONG): protocol for a randomised controlled feasibility trial
Waddell A,1 Denton F,1 Powell R,1,2 Birkett S,3 Broom D,1 Imray C,4 McGregor G,1,2,5 Harwood AE1
Plain English Summary
Why we are undertaking the research: Peripheral artery disease is a common problem where the blood vessels in the legs are narrowed by fatty deposits. Supervised exercise programmes are recommended to help treat this condition, as they can reduce leg pain and improve fitness. However, not many people are able to access these programmes typically because of barriers including travel burdens, time constraints or other commitments. As an alternative, researchers are developing home-based programmes which do not require people to travel to centres for their sessions. In the UK and to the author’s knowledge, there are not many well researched home-based programmes available for people living with peripheral artery disease.
What we aim to do: We plan to undertake a study to see how feasible our home-based programme is. People with peripheral artery disease will either be asked to continue with their normal routine or will be prescribed an exercise programme, with an activity watch to monitor physical activity. This programme will include increasing the number of steps walked each day, an exercise circuit (twice a week) and a telephone support call with a member of the research team to discuss their progress or lack of progress. Questionnaire responses, blood samples, walking ability, muscle strength and the amount of daily exercise will be compared between the two groups at the start of the 12-week programme, at the end, and 12 weeks after the programme has finished. By doing this study, we will be able to refine our home-based exercise programme so that it can be tested on a larger scale to see if it is a good option for people with peripheral artery disease who may not be able to attend a supervised exercise programme.
Trial Registration:
ClinicalTrials.gov, NCT05059899. Registered on 17 September 2021
Introduction
Peripheral artery disease (PAD) refers to the progressive occlusion of the arteries supplying the lower limbs1 and affects over an estimated 236 million people worldwide.2 Sustained ischaemia to the lower limbs, resulting in an oxygen supply/demand imbalance, can lead to a symptomatic presentation of PAD, characterised by exertional cramp-like leg pain known as intermittent claudication (IC).3
Current guidelines recommend supervised centre-based exercise therapy (SET) to improve walking performance and quality of life in those with IC.4-6 UK-based guidelines suggest exercise to the point of maximal claudication pain.4 Despite all the evidence for the effectiveness of SET, access, adherence and uptake is poor because of travel burdens, time commitments and lack of motivation.7,8
To improve accessibility and participation, there is a growing interest in home-based exercise programmes (HBEP). However, a recent UK-based survey found that only 48% of vascular services were able to offer SET, and of these only 30% provided any home exercise advice (alongside existing SET services), in the form of verbal recommendations, exercise booklets and pedometers.7 This issue has been heightened during the COVID-19 pandemic, as sites delivering SET have had to provide home-based alternatives or face cancelling services completely. These services were therefore forced to quickly transition into HBEPs without the experience and a limited evidence base, highlighting the importance of developing HBEPs for UK patients.7 A recent systematic review has shown HBEPs to be a safe option for people with IC, with an all-cause adverse event rate of one per 36,953 patient-hours.9
HBEP interventions vary considerably in relation to frequency, modality, location (inside the home, outdoors in the community or in leisure centres)10 and level of support provided. Technology used to remotely monitor progress, such as wearable activity trackers, has been shown to increase the efficacy of HBEPs, equivalent to that of SET.11 Some programmes mimic SET in a home setting while others, including UK-based studies, aim to increase daily physical activity with step goals or generic walking advice.12-18
An example of a structured prescription of home-based exercise includes walking at a self-selected pace three days per week for 20–45 minutes over 12 weeks.14 Adherence was monitored with a step activity monitor, resulting in significant improvements in walking performance. An example of a step-based programme had participants use a Fitbit to increase physical activity by 2500 steps per day over baseline for one month, increasing to 3750 and 5000 for months 2 and 3, respectively.12 Improvements were seen in walking performance and cardiorespiratory fitness. However, simply promoting an increase in step count or physical activity is not sufficient. Results from the LITE trial showed walking performance improved more by sustained individual bouts of physical activity rather than just increased total activity.19 Further, progressing activity with a blanket step goal for all participants (such as an additional 1000 steps) does not account for individual differences. Therefore, in addition to increasing daily activity, more structured individualised walking programmes are required.
In addition to walking, resistance training may also improve functional ability; however, to our knowledge, only one study has assessed this as part of a HBEP.20 Incorporating resistance training into a walking programme may improve strength and balance whilst also increasing adherence by providing variety, both of which may translate to tangible functional improvements and gains in quality of life. There is therefore potential for a HBEP that utilises all three of these elements: increasing physical activity behavior with a step goal, while providing structured exercise prescription that includes walking to induce claudication pain and resistance exercises. The WALKSTRONG protocol was therefore developed taking this into consideration.
Before a definitive randomised controlled trial (RCT) can assess the clinical and cost effectiveness of our HBEP, the intervention must first be refined. Therefore, the aims of the present feasibility study are to:
1. Assess the feasibility of conducting a RCT of community walking and home-based circuit training in people with IC.
2. Measure recruitment and attrition rates.
3. Measure protocol adherence and safety.
4. Conduct semi-structured interviews with intervention completers, drop-outs and decliners to assess acceptability, facilitators and barriers.
5. Explore changes in walking performance, functional ability, quality of life and markers of systemic inflammation and vascular remodelling.
Methods
Study design
WALKSTRONG is a prospective assessor blind randomised controlled feasibility study. Thirty adults over the age of 18 years with IC will be randomly assigned to either a 12-week home-based exercise intervention or a usual care control group. This protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines.21
The study design is illustrated in Figure 1.
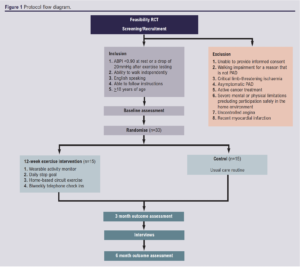
Study setting
The study is being undertaken at the Centre for Exercise & Health, Coventry in collaboration with University Hospitals Coventry & Warwickshire (UHCW). The study will take place from January 2022 to July 2024 and is sponsored by Coventry University. Funding to deliver the RCT is provided as part of a PhD scholarship for the principal researcher (AW) from Coventry University.
Study registration and ethical approval
Ethical approval was granted by the local research ethics committee (Coventry & Warwickshire REC: 21/WM/0208) on 14 December 2021 and the study will be conducted in accordance with the Declaration of Helsinki 1975. The study was prospectively registered on ClinicalTrials.gov (NCT05059899).
Inclusion and exclusion criteria
Participants with a confirmed diagnosis of IC will be recruited. The inclusion and exclusion criteria are listed below:
Inclusion criteria
• >18 years of age.
• Ankle/brachial index (ABPI) <0.9 at rest or a drop of 20 mmHg after exercise testing.
• Ability to walk independently without walking aids.
• English speaking.
Exclusion criteria
• Unable to provide informed consent.
• Walking impairment for a reason that is not related to PAD.
• Critical limb-threatening ischaemia or asymptomatic PAD.
• Active cancer treatment.
• Severe mental or physical limitations precluding participation safely in the home environment as defined by American College of Sport Medicine.22
• Unstable angina.
• Recent myocardial infarction (within the previous month).
Study procedures
Patients who are potentially eligible will be referred to the research team by the clinical care team, having been initiated on best medical treatment by the referring clinician. If eligible, potential participants will be provided with a WALKSTRONG information sheet and be contacted to assess willingness to participate. Those who decide to participate will attend a baseline visit to confirm eligibility and provide informed consent. On completion of the baseline visit, participants will be randomised into the intervention or control group. Participants will be informed that they are free to withdraw from the trial at any time without providing a reason. If a participant’s circumstances change such that they become ineligible to participate, the principal investigator reserves the right to withdraw the participant from the programme. Participants will exit the trial if they finish the intervention and complete outcome assessments at 12 and 24 weeks, or if they request to withdraw, or die.
Randomisation
Participants will be randomised in a 1:1 ratio using random permuted blocks via a secure computerised randomisation programme, maintaining allocation sequence concealment (Sealed Envelope: https://www.sealedenvelope.com). Participants will be randomised following baseline assessment. At subsequent visits, data will be collected by a different assessor who will be unaware of group allocation.
Control group
Participants allocated to the control group will not be given any intervention and will receive standard care which will include smoking cessation support and basic unstructured exercise advice by providing them with a leaflet from the British Heart Foundation. This includes information on the benefits of being physically active, what counts as activity, and how much physical activity should be undertaken each week. To ensure equity between participants, following the completion of the trial, those in the control group will be offered a Fitbit watch and the opportunity to complete the HBEP.
Exercise intervention
Participants assigned to the HBEP will be given a Fitbit Charge 4 activity monitor (https://www.fitbit.com) free of charge and will be required to download the Fitbit app using a study-specific email address provided to them. They will be asked to use the Fitbit to reach a personalised daily step goal, at their own pace, which will be determined based on baseline daily step count (measured over one week with an accelerometer). They will be initially asked to increase their daily step count by 10% above what they achieved at baseline. They will also be provided with a set of circuit exercises to be completed in their home twice per week, lasting approximately 45 minutes – as many UK centres prescribe exercise 1–2 days a week and a minimum of two resistance sessions a week is recommended.7,23 This will involve a light warm-up period of 10 minutes including rhythmic pulse raising movements, joint mobilisation and passive stretches. This will be followed by a circuit, separated into six stations of resistance exercises. These include shoulder press, calf raise, wall press, sit-to-stand, bent over row and lunges, interspersing upper and lower body exercises. The aim is to complete two minutes at each station at 11–14 on the Borg scale of perceived exertion. Each station is separated by walking on the spot at an intensity to induce severe claudication pain for two minutes (but may be longer if severe pain is not reached in this time). The circuit will be followed by a 10-minute light cool down. Participants will be asked to record each circuit workout using an exercise logbook, noting the number or repetitions and weights used as well as leg pain during each walking bout.
The exercise intervention was designed based on current best practice guidelines for the management of PAD,4,6 evidence from previous research11 and informal public and patient involvement (PPI) discussions (see Appendix 1 online at www.jvsgbi.com). Findings from PPI discussions were that patients were keen to have a home-based alternative to SET, were interested in using an activity watch to visualise improvements, were happy to complete resistance exercise if given appropriate instructions and that check-ins should be biweekly.
Researchers will monitor the compliance of participants with their daily step goal by accessing their Fitbit data weekly. Every two weeks, participants will be contacted by telephone, providing an opportunity to discuss any issues or questions. Daily step count, exercise intensity and frequency will be discussed, with a mutual decision being made on progression or regression.
Outcome measures
Primary outcomes
The primary outcomes for this study are feasibility and acceptability, assessed via recruitment, attrition, adherence to the protocol, adverse events and participant feedback.
Recruitment: calculated by dividing the number of eligible candidates by the number who consent to participate.
Attrition: established as discontinuation of the intervention and loss to follow-up.
Adherence: assessed by monitoring logbook entries for the exercise sessions and recorded physical activity data will assess an individual’s engagement with the intervention. This will include the percentage of step goals achieved, percentage of circuit sessions completed, partially completed and percentage completed at the required intensity. Where possible, reasons for drop out will be recorded to assess the suitability of the protocol.
Adverse events: reported in accordance with the principles of Good Clinical Practice, with the relatedness of the event to the intervention being assessed by the research team in collaboration with the clinical care team. Serious adverse events will be reported to the relevant ethics committee and sponsor. In the event of harm arising to participants as a result of study management, design or conduct, Coventry University insurance and indemnity policies will apply.
Acceptability: assessed by conducting semi-structured interviews. Participants who have completed the trial, as well as those who drop out or decline to take part, will be offered the opportunity to take part in a semi-structured interview either in person, over the telephone or via video conference with a member of the research team. With permission from participants, conversations will be audio recorded and transcribed verbatim. The interviews will be conducted using a topic guide to have consistency across all participants; however, they will be flexible to allow for discussion. Interviews will explore participants’ opinions and experiences with the exercise programme, and any criticisms or factors that limited or enabled participation. Interviews will last approximately 30–60 minutes.
Secondary outcomes
Pain-free and maximal walking distance: This will be determined via a 6-minute walk test and graded treadmill test. The 6-minute walk test will be conducted in accordance with the guidelines from the American Thoracic Society.24 This involves a 30 m course indoors, along a flat surface. The course will be marked every 3 metres with cones at each end. After the patient has rested, they will be instructed to walk as far as they can within the time limit, receiving standardised encouragement every minute. Participants can stop walking during the test for rest; however, the timer will not be stopped. Speed gates will be placed at each end of the course to measure walking speed.
The graded treadmill test (Gardner protocol) involves walking at a constant speed of 2 mph, at a 0% incline grade, which increases by 2% every 2 minutes. Participants will be instructed to indicate when claudication pain begins, giving pain-free walking distance (metres), and when claudication pain becomes too severe to continue walking, giving maximal walking distance (metres).25 Adequate time will be provided between each test to ensure recovery.
Grip strength: Participants will be asked to hold an isometric dynamometer in their hand, with the handle being adjusted so that the base rests in the heel of their palm. Participants should be seated, resting their arm on the chair arm. When they are ready, they will be asked to squeeze the dynamometer with maximal effort for 5 seconds. The test will be repeated three times on each hand with full recovery between each effort, recording the greatest value, as described by the Southampton protocol and American Society of Hand Therapists.26
Physical activity behaviour: determined by giving participants a research grade accelerometer (ActiGraph GT3X) to wear for one week. Participants will be asked to go about their normal activity and should wear the device continuously, only removing it when washing or sleeping. The ActiGraph GT3X is a reliable, gold standard accelerometer for measuring physical activity in adults under free-living conditions.27
Quality of life: will be recorded by assessing responses to three questionnaires. Disease-specific quality of life will be measured using the VascuQol questionnaire, a validated and standardised intermittent claudication-specific questionnaire.28 The 36 Item Short Form survey will also be used. It is a well validated, generic health status questionnaire which provides a score on eight domains of health: bodily pain, mental health, vitality, physical and social functioning, physical and emotional roles and general health.29 Answers to the EQ-5D-5L questionnaire will also be collected.30,31
Blood sampling: blood will be drawn from participants at each visit by a member of the research team trained in venepuncture using standardised operating procedures. Blood samples will be centrifuged and serum aliquoted and stored at –80°C until they are analysed. Assays will be performed by a member of the research team at Coventry University. Markers of systemic inflammation and vascular remodelling will be measured via serum C-reactive protein, interleukin-6, tumour necrosis factor-alpha and vascular endothelial growth factor using an enzyme-linked immunosorbent assay with each sample measured in duplicate.
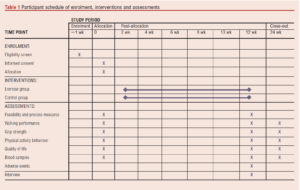
An overview of the participant pathway through the study is shown in Table 1.
Sample size
As this is a feasibility trial, a power calculation was not completed. A general recommendation for feasibility studies is to include at least 30 participants, and it has been suggested that samples between 24 and 50 are sufficient to calculate a standard deviation of an outcome that can then be entered into a formal power calculation for a full-scale RCT.32-34
Data collection, storage, management and monitoring
Data will be collected by the principal investigator and other named and trained co-investigators at all time points. Data will be anonymised and given a study code. All other data will be stored securely at The Centre for Exercise and Health and/or saved on encrypted computer drives. Only members of the research team will have access to the dataset. Data will be securely archived after study closure and stored for up to 5 years, after which it will be destroyed. All data will be stored and managed according to Coventry University’s confidentiality and data protection policies. No formal data monitoring committee will be formed. The study will be regularly monitored by the research team members, led by the chief investigator (AH), throughout the study period.
Data analysis
All quantitative statistics will be presented as mean ± standard deviation (SD) unless otherwise stated. Participant data will be analysed in accordance with the intention-to-treat model, analysing according to the group to which they were randomised, regardless of the intervention they received.
Descriptive statistics will be used to describe the two groups at baseline, being assessed for normality via the Shapiro–Wilks test. To assess heterogeneity of the randomised groups, outcomes will be compared using independent t-tests or Wilcoxon signed-rank tests. All statistical analysis will be undertaken using IBM SPSS Statistics software.
Exploratory analysis
Data for walking performance, functional ability, physical activity, blood samples and quality of life will be assessed at baseline, week 12 and week 24. Presuming data are normally distributed, differences between means will be assessed using a mixed model repeated measures ANOVA with group allocation as the between-subject factor and time as the within-subject factor. Data will be analysed to assure they meet the assumptions of an ANOVA test and post hoc analysis will be undertaken on significant differences between group means. Partial eta squared will be reported as effect size. Statistical significance will be inferred if p<0.05. Results of all analyses will be interpreted and reported with the knowledge that the study is a feasibility trial that has not undergone a formal sample size calculation, and so is exploratory.
Qualitative data obtained during the semi-structured interviews will be managed and analysed using the qualitative software package (NVIVO). Qualitative data will be analysed using thematic analysis.35 This is an inductive and iterative approach, in which themes will be derived from the data and agreed by the researchers following triangulation. Collection and analysis of the data will occur simultaneously.36
Dissemination of study findings
Trial participants will be provided with a summary of the results of the study. We will seek to publish results in reputable peer-reviewed journals and present the findings at relevant conferences. The results of the study and participant or sponsor information will not be passed on to any third party without gaining participant and sponsor consent.
Discussion
Supervised exercise alongside optimisation of best medical treatment and reduction of risk factors (ie, smoking) is currently first-line treatment for people with IC.5 There has been a surge in research investigating the effectiveness of home-based alternatives to account for the underutilisation and lack of availability of SET in clinical practice. Typically, these programmes follow a similar structure to SET, with intermittent walking to the onset of moderate to maximal claudication pain, 3–5 days per week, progressively increasing the duration.14,37 There is also growing interest in programmes that promote daily physical activity. To account for the lack of supervision, in-person or virtual check-ins are frequently implemented to discuss progress and alter intensity.
WALKSTRONG aims to investigate the feasibility of implementing a 12-week exercise intervention that encourages increases in physical activity behaviour and undertaking a home-based exercise circuit in people with IC. This will allow for the refinement of the protocol which can be used in a fully powered RCT to assess the effect of the programme on walking performance. The WALKSTRONG study is unique as it aims to incorporate home-based resistance training exercises with structured walking. Additionally, an increase in daily steps as a percentage of baseline rather than as a set number of steps will be implemented to promote physical activity. The programme aims to expand the ability of healthcare systems to provide a remote exercise programme to those unwilling or unable to take part in SET. A limitation is that there may be some socioeconomic bias in sample selection, as a smartphone will be needed to synchronise with the activity monitor to provide step data.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2022;1(3):93-99
Publication date:
May 11, 2022
Author Affiliations:
1. Centre for Sport, Exercise and Life Sciences, Institute of Health and Wellbeing, Coventry University, Coventry, UK
2. Department of Cardiopulmonary Rehabilitation, Centre for Exercise & Health, University Hospitals Coventry & Warwickshire NHS Trust, Coventry, UK
3. School of Sport and Health Sciences, University of Central Lancashire, Preston, UK
4. Coventry NIHR Clinical Research Facility, University Hospitals Coventry & Warwickshire NHS Trust, Coventry, UK
5. Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick, Coventry, UK
Corresponding author:
Mr Alexander Waddell
Centre for Sport, Exercise and Life Sciences, Institute of Health and Wellbeing, Coventry University, Coventry CV1 2DS, UK
Email: alexanderjrwaddell@ gmail.com











