WINNING ESSAYS
Rouleaux Club Winning Essays 2022
The Rouleaux Club run an annual essay competition to help promote interest in vascular surgery. Entrants are asked to write 1,500 words on one of three topics selected by the RC Executive. The essays are marked by the committee and the prizes are awarded to the best essay at the annual Vascular Society meeting. There are two prize categories, one for medical students and another for junior doctors. Following the Vascular Societies GB&I Annual Scientific Meeting, the winning essays will be published in the journal.
STUDENT CATEGORY
Should the threshold for elective AAA repair be raised to 7 centimetres?
Hailey Rees, University of Dundee
An abdominal aortic aneurysm (AAA) is a degenerative condition caused by the abnormal dilatation of the abdominal aorta.1 In the UK, there is a prevalence of 1.3% of men with an AAA over the age of 65 years, and a death rate of 3,000 patients every year.2,3 As the mortality from a ruptured abdominal aortic aneurysm approaches 80-90%, the single goal of elective surgical repair is the prevention of this complication.4 In contemporary UK practice, a national screening programme facilitates the identification of patients with an abdominal aortic aneurysm and has been demonstrated to reduce deaths from aortic aneurysm rupture.3,5 In current practice, aortic diameter remains the primary trigger to consider elective repair with the “threshold” for elective aneurysm repair in asymptomatic aneurysms being an aortic diameter of 5.5cm and above.3,6 Whilst this “threshold” is based on a series of randomised control trials (including UK Small Aneurysm Trial and Aneurysm Detection and Management Trial),7,8 which demonstrated reduced risk of rupture and patient mortality, it remains (to an extent) arbitrary. This is due to limited data comparing the effects of surveillance in aortic diameters >5.5cm to that of surgical intervention, and whether the risk of aneurysm rupture is significant enough to warrant surgical repair at smaller diameters.9 Therefore, it comes into question whether or not the current repair threshold by studious default should be raised to larger diameters such as that of 7cm.
In current literature, determining the risk of AAA rupture in correlation with pre-operative aortic diameter has proven to be significantly challenging.6 The general consensus within clinical practice is an increasing aortic diameter would increase a patient’s risk of rupture and mortality.9 Whilst this remains true, it is important to consider the relative risk of rupture with increasing diameter and whether this risk is significant enough to justify operative management at larger diameters. Grima et al., aimed to determine the relationship between mean diameter of intact AAAs (iAAA) for elective repair with rupture rates (rAAA) in 9 different countries6. This incorporated a wide variation in mean aortic diameter, most of which, beyond the recommended threshold (µ = 6.2cm in males, µ = 5.9cm in females).6 Results found no statistical significance between reported mean iAAA diameter and rAAA repair rate. A meta-analysis by Parkinson et al, investigated untreated aneurysms in patients declared unfit for surgical repair with percentage risk of rupture per year. It was concluded that cumulative risk of rupture was 3.5% in aneurysms 5.5-6.0cm in diameter, 4.1% between 6.1-7.0cm, and 6.3% for >7.0cm. Despite showing a generalised increase in rupture rate with increasing diameter, the rupture rates were lower than which is commonly reported in literature.10 However, determining at which size the rupture risk is significant enough remains unclear. Reported by Lo et al, the size of an AAA at point of rupture in male patients was 7.9cm and for females, 7.1cm.11 Retrospectively, such studies demonstrate the relative risk of rupture in patients with aortic diameters above the recommended threshold could be managed conservatively under surveillance without surgical intervention. This is due to the lack of statistically significant relationships between high rupture rates in larger aneurysms and the low mortality rates in aneurysms measuring 6.1-7.0cm.
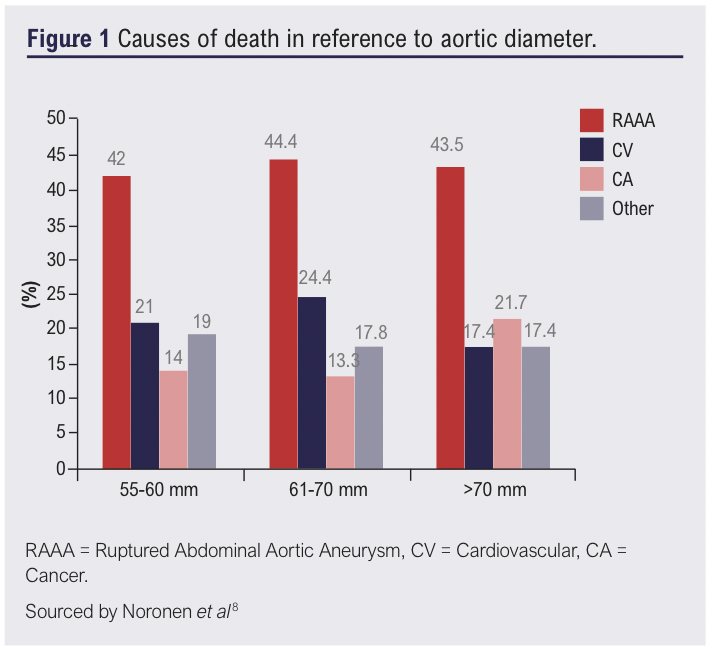
Evidence to the contrary deems mortality risk of larger aneurysms too great for the clinical threshold to be raised above 5.5cm. A retrospective study by Noronen et al, analysed mortality rates in patients with aneurysms that met elective repair criteria but not operative criteria.9 Of the 798-patient cohort, they found with increasing aneurysm diameter, there was a decrease in cumulative survival more so in aneurysms sized >6.1cm. Furthermore, the median time of aneurysm rupture reduced by 50% when aortic diameter reached >6.1cm demonstrating a more probable likelihood of rupture in larger aneurysms.9 It has also been commonly reported in many studies that patients with an AAA are likely to die from other causes, than they are by aneurysm rupture. Noronen et al, further analysed causes of death in accordance with aortic diameter as shown in Figure 1.9 Independent of aortic diameter, the leading cause of death was that of aneurysm rupture. Therefore, it could be suggested that elective repair in larger aneurysms would provide significant benefit to patients and reduce mortality secondary to rupture. By reducing disease burden through early intervention when diameters meet current guidance, the likelihood of rupture is reduced before the risk poses too great a threat or deemed surgically untreatable.
Emergence of the COVID-19 pandemic profoundly affected services supplied by the UK National Health Service. This led to substantial delay in elective AAA repairs.12 New guidance by the UK National Joint Vascular Implementation Board came to light to postpone elective AAA procedures and re-evaluated the diameter threshold for repair.13 This decision involved balancing risks around COVID-19 and operative mortality, with rupture risk.14 Recommendations published were to delay elective repair by 12 months in aneurysms measuring 5.5-6.0cm, and 6 months if 6.0-7.0cm.12 McGuinness et al, evaluated through probabilistic sensitivity analysis, the potential harm delayed AAA repair could have on patients.14,15 This study reported a probability of survival increase with immediate operative management in aneurysms sized 5-6.9 cm, compared with delayed repair. Moreover, aneurysms >7cm reported similar, however the survival probability distribution was lower compared to aneurysms of 5-6.9cm.14 Therefore, a delay in elective repair of aneurysms could pose increasing risk of harm to patients with larger aneurysms. However, reported aneurysm sizes met both the current threshold criteria for elective repair and larger. Evidently, despite surgical interventions improving probability of survival, patients were living with much larger aneurysms before repair, and showing increasing benefit of surgical management at these diameters.
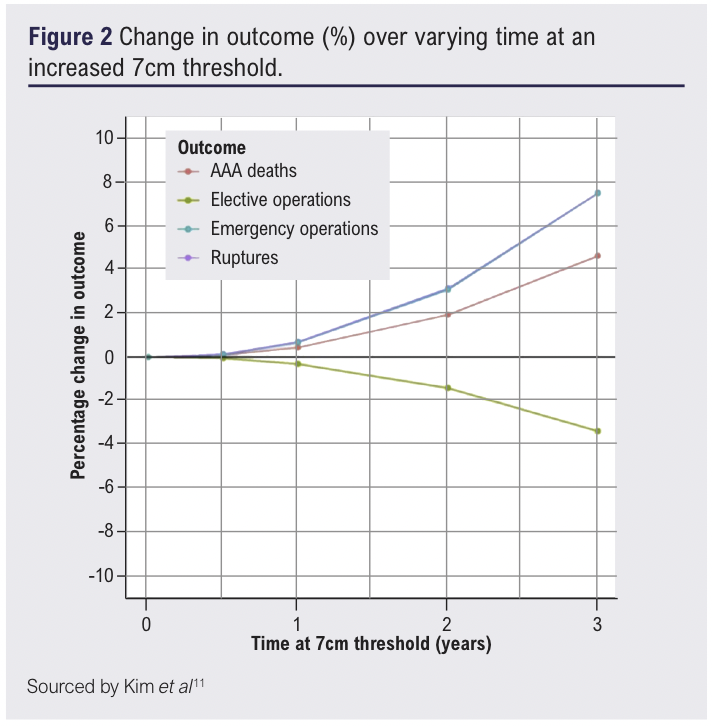
It could be argued that had there been no delay in surgical intervention, this could further improve probability survival at smaller aneurysm sizes. It is important however, to put this probability into perspective and quantify the risk larger aneurysms at 7cm have on mortality. Kim et al, evaluated the impact delayed services had on patient mortality and the change of elective threshold to 7cm during the pandemic.12 This study noted a delay of 1 year for elective repair contributed a modest increase in mortality of 0.4% compared to two years, where mortality increased to 1.9% (Figure 2). The risk of rupture increased in a similar fashion exhibiting a 0.7% increase in mortality after one year and 3.1% at two years (Figure 2).12 This established patients could potentially live under surveillance at larger diameters with a relatively low risk of rupture and mortality, but within a shorter time period of 2 years.
Another consideration is the post-operative risk of elective repair and whether operative management at the recommended threshold is causing patients more harm than good. Comparing effects of surveillance versus elective repair of aneurysms <5.5.cm as demonstrated by the UKSAT, ADAM, CAESAR and EVAR 2 trials, researchers could not demonstrate any benefit for early elective repair when compared with surveillance alone.1,7,8,16,17 Such literature provided evidence to support the current threshold. Despite this, there is limited evidence to support elective repair over surveillance in larger aneurysms closer to that of 7cm. Whilst patients undergoing operative management are at risk of complications such as infection or haemorrhage, the EVAR 1 trial demonstrated that EVAR exhibited inferior late survival benefit compared with OAR.17 Patients with larger aneurysms of >6.0cm also tend to be older, have reduced surgical fitness and unfavourable neck anatomy.18 Zarins et al, investigated the effects of EVAR after a 5-year period in both small (<5.0cm) and large (>6.0cm) aneurysms. Results of this study found patients with larger aneurysms, showed an increased risk of aneurysm rupture and mortality, surgical conversion and suffered more aneurysm-relate deaths post-EVAR compared to the small aneurysm cohort.19 Whilst this in hindsight would favour the recommended threshold, it could be argued that increasing this threshold to 7cm would prolong the incidence of post-operative complications as this would be offered further down the line. However, finding the balance between surgical fitness of a patient, with the benefits of surgical repair against mortality and rupture rates is difficult to quantify.
Considering factors discussed, the implications larger aneurysms could have on patient’s lives remains challenging. It comes into question whether patients are undergoing unnecessary operative repair at smaller diameters. Such parameters could increase risk of secondary repairs and complications compared to monitoring when the risk of rupture is not as high as previously thought. However, many patient factors have to be carefully considered when clinicians offer elective repair. More research is required to establish long-term effects of surveillance and repair in larger aneurysms to support a change in threshold. This would provide evidence to build risk assessment criteria by incorporating a patient’s clinical risk factors with their risk of mortality over varying time.
References
1. Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK endovascular aneurysm repair (EVAR) randomised controlled trials: Long-term follow-up and cost-effectiveness analysis. Health Technology Assessment 2018;22(5):1–132. https://doi.org/10.3310/hta22050
2. Whittaker JD, Meecham L, Summerour V, et al. Outcome after turndown for elective abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2017;54(5):579–86. https://doi.org/10.1016/j.ejvs.2017.07.023
3. Earnshaw JJ. The indication for elective repair of abdominal aortic aneurysm should be reviewed. Eur J Vasc Endovasc Surg 2021;61(1):7–8. https://doi.org/10.1016/j.ejvs.2020.09.001
4. Hoornweg LL, Storm-Versloot MN, Ubbink DT, Koelemay MJW, Legemate DA, Balm R. Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2008;35(5):558–570. https://doi.org/10.1016/j.ejvs.2007.11.019
5. Scott RAP. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. The Lancet 2002;360:1531-9.
6. Grima MJ, Behrendt C-A, Vidal-Diez A, et al. Editor’s choice – assessment of correlation between mean size of infrarenal abdominal aortic aneurysm at time of intact repair against repair and rupture rate in nine countries. Eur J Vasc
Endovasc Surg 2020;59(6):890–7. https://doi.org/10.1016/j.ejvs.2020.01.024
7. Powell JT. Final 12-year follow-up of surgery versus surveillance in the UK small aneurysm trial. British Journal of Surgery 2007;94(6):702–08. https://doi.org/10.1002/bjs.5778
8. Lederle FA, Wilson AE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002;346: 1437–44. https://doi.org/10.1056/NEJMoa012573
9. Noronen K, Laukontaus S, Kantonen I, Lepäntalo M, Venermo M. The natural course of abdominal aortic aneurysms that meet the treatment criteria but not the operative requirements. Eur J Vasc Endovasc Surg 2013;45(4):326–31. https://doi.org/10.1016/j.ejvs.2012.12.019
10. Parkinson F, Ferguson S, Lewis P, Williams IM, Twine CP. Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. J Vasc Surg 2015;61(6):1606–12. https://doi.org/10.1016/j.jvs.2014.10.023
11. Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013; 57(5):1261–8.
12. Kim LG, Sweeting MJ, Armer M, Jacomelli J, Nasim A, Harrison SC. Modelling the impact of changes to abdominal aortic aneurysm screening and Treatment Services in England during the COVID-19 pandemic. PLOS ONE 2021;16(6). https://doi.org/10.1371/journal.pone.0253327
13. Guidance on resumption of vascular surgery [Internet]. Vascular Society. 2020 [cited 10 Oct 2020]. Available from: https://www.vascularsociety.org.uk/professionals/resources/covid19
14. McGuinness B, Troncone M, James LP, Bisch SP, Iyer V. Reassessing the operative threshold for abdominal aortic aneurysm repair in the context of COVID-19. J Vasc Surg 2021;73(3):780–8. https://doi.org/10.1016/j.jvs.2020.08.115
15. Deery SE, Schermerhorn ML. Should abdominal aortic aneurysms in women be repaired at a lower diameter threshold? Vasc Endovasc Surg 2018;52(7): 543–47. https://doi.org/10.1177/1538574418773247
16. Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E. Comparison of surveillance versus aortic endografting for small aneurysm repair (caesar): Results from a randomised trial. Eur J Vasc Endovasc Surg 2011;41(1):13–25. https://doi.org/10.1016/j.ejvs.2010.08.026
17. Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (evar trial 1): A randomised controlled trial. Lancet 2016;388(10058):2366–74. https://doi.org/10.1016/S0140-6736(16)31135-7
18. Hye RJ, Janarious AU, Chan PH, Cafri G, Chang RW, Rehring TF, Nelken NA, Hill BB. Survival and reintervention risk by patient age and preoperative abdominal aortic aneurysm diameter after endovascular aneurysm repair. Annals of Vascular Surgery 2019;54:215–25.
19. Zarins CK, Crabtree T, Bloch DA, Arko FR, Ouriel K, White RA. Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome? J Vasc Surg 2006;44(5):920–30. https://doi.org/10.1016/j.jvs.2006.06.048
DOCTOR CATEGORY
Should the threshold for elective AAA repair be raised to 7 centimetres?
Faraaz Khan, Thames Valley
Introduction
Determining the threshold for elective AAA repair is a balance between rupture risk with likely mortality and elective operative risk. Repair options include open surgical repair (OSR) and more recently endovascular aortic repair (EVAR). The clinical landscape of AAA is ever changing with decreased rates of ruptured AAA (rAAA) and improved operative outcomes. As such the balance between surveillance and intervention must be reassessed over time with particular focus on whether intervention only improves AAA-related mortality and not all-cause mortality which is especially pertinent in an aging population. In parallel, we may begin to observe a shift away from a single metric to ensure inclusivity to all patient demographics and strive for personalised medicine.
Current practice and evidence
The European Society of Vascular Surgery (ESVS) published guidance on the management of AAA, including the indications for elective repair (Table 1).1
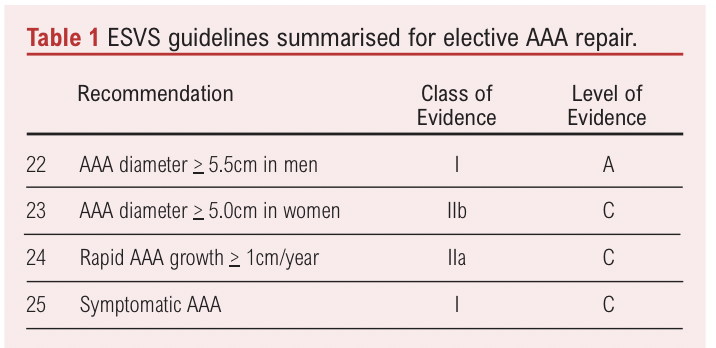
The thresholds established for elective intervention in men are founded on the results of four clinical trials. With comparison to surveillance for aneurysms <5.5cm in diameter, the UKSAT and ADAM trials evaluated OSR. The CAESAR trial and PIVOTAL study evaluated EVAR.2-5 A review concluded that none of the trials individually or collectively found a significant difference in outcomes between surveillance or intervention for long term survival or quality of life. Based on lack of clinical benefit and greater cost of intervention over surveillance the minimum threshold for men has since been well-established at 5.5cm for elective repair.
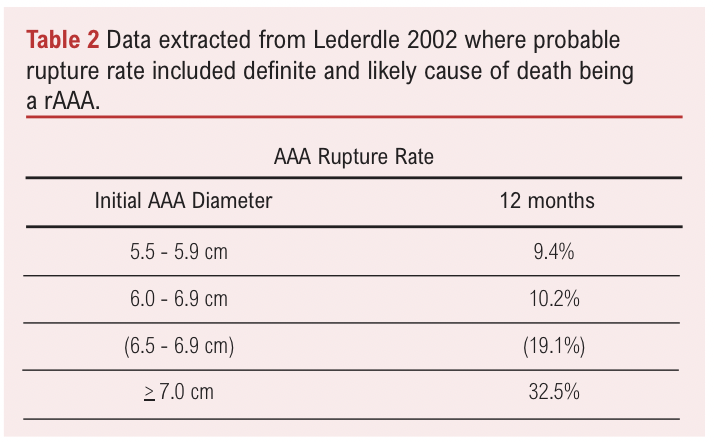
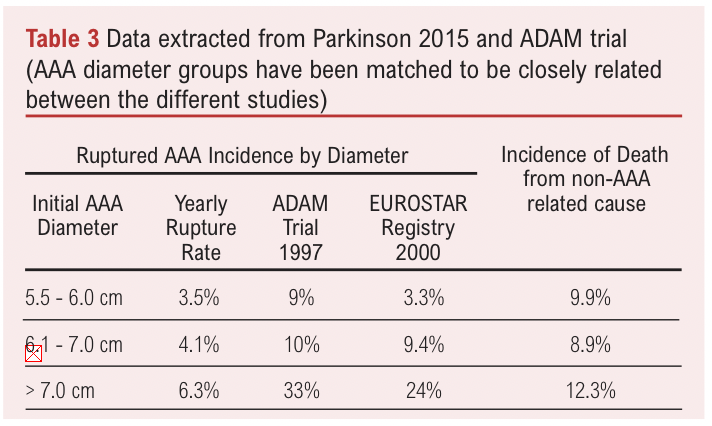
The origin of the 5.5cm threshold for men originates from studies from the early 2000s that found that the rAAA rate was associated with AAA diameter and importantly, a high AAA-related mortality rate for patients with an AAA diameter >5.5cm (Table 2).6 Notably, the mortality rate in patients with an AAA diameter of 5.5-5.9cm (9.4%) was approximately 10-fold higher than that of patients with an AAA diameter of 4.0-5.5cm as reported in the UKSAT and ADAM trial. However, this is not an accurate comparison as the patient demographics are unequal, for example co-existing incidence of hypertension (66.2% vs 40%), and the clinical trials excluded patients not fit for surgery.
A review of 11 studies found much lower rAAA rates by AAA diameter in patients unfit for surgery in contrast to earlier reports such as the ADAM trial (Table 3).3,7 It is likely the rupture rate has decreased over the past few decades likely due to changing patient demographics and better management of comorbidities (Table 3).8 As the rupture rate and risk of AAA-related mortality is seemingly lower than that of non-AAA related causes, this calls into question whether the current elective threshold should be increased. One of the included studies monitored the progress of patients turned down for elective AAA repair over 10 years. There was little difference in the rate of rupture and non-rupture related death in patients with an AAA of 5.5-5.9cm while differences were noticed in patients with an AAA >6cm by 6 months.9
Whether outcomes of elective repair are dependent on aneurysmal size will also influence the threshold for elective repair. A retrospective study found that those with smaller aneurysms (<5.5cm) were more likely to survive at the 1 year (93% vs 88%) and 6 year (64% vs 47%) timepoints even after adjusting for age and sex.10 Multiple reports of comparisons of elective repair for outcomes of small (<5.5cm) or large AAAs and have found reduced odds for all-cause mortality, in addition to freedom from complications for small aneurysms.11-14 This collection of evidence suggests that the decision to increase the elective repair threshold is not only dependent on risk of rupture but also the differing clinical outcomes when operating on larger aneurysms.
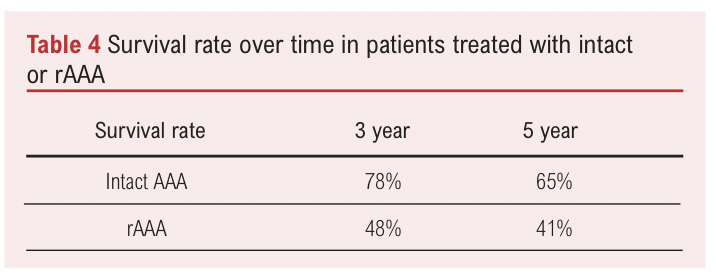
Contrastingly, improvements in perioperative management of elective and rAAA repair would allow for a decrease or increase in the elective threshold respectively. Improvements in repairing rAAAs could mean that patients can be kept under surveillance for longer to reach a higher elective threshold in the knowledge that if a rupture did occur the likelihood of success is acceptable. Patient post-op and perioperative mortality post emergency rAAA repair has decreased significantly, based on data from the NSQIP database over the 2005–2011 period.15 It is possible the increased widespread use of EVAR and new techniques have contributed to this shift. A retrospective study of 152 rAAA and 467 elective AAA repair patients found that rAAA patients had a significantly higher 30 day mortality rate (32.9% vs 3.4%) but after this period a similar change in survival rate over time (Table 4).16 Improvements to perioperative outcomes in rAAA repair, specifically to match that of elective AAA repair would suggest increasing the elective repair threshold. However, it still remains that around half of patients with rAAA do not make it to hospital for emergency repair.17
Studies have reported conflicted results for the use of use of antiplatelets and beta blockers to benefit survival in elective repair.18,19 Statin use however has been shown to reduce elective AAA operative risk and improve long term survival.20 However, there is no evidence of medical intervention that can prevent AAA growth.21 Further improvements to elective outcomes may indicate a decrease in threshold would be appropriate.
National screening
UK and Sweden screening programmes invite men over 65 years old for an ultrasound assessment using a 5.5cm threshold. While the Chichester (UK) study did not find a significant decrease in AAA related mortality, the Sweden screening programme did so successfully.22,23 A 2014 review of 4 screening studies in different locations found that AAA screening did not reduce all cause mortality at any time point in the 15 years of data collection available.24 However, when additional follow up data from the Western Australia trial was subsequently included there was small but significant reduction in all cause mortality (RR 0.986) and AAA-related mortality.25 NHS AAA screening is cost effective with a reported £7370 per quality-adjusted life-year (QALY) gain which is comfortably within the NICE threshold.27,27 However, with decreasing AAA prevalence, methods to maintain cost-effectivness such as lengthening surveillance intervals has been evaluated but it is unclear if the marginal benefit is sufficient to change clinical practice.28 Increasing the elective repair threshold would likely decrease cost of elective surgeries but at the expense of an increased rAAA rate.
Demographic pitfalls of current practice
A major limitation of the studies discussed in this article is that their patient demographic is predominantly western men. The RESCAN meta-analysis found that women have up to 4 times increased likelihood for rAAA compared to men and at a smaller average diameter (5.0 vs 6.0cm).29,20 The AAA diameter is also inferior to the aortic size index (ASI, a ratio between AAA diameter and body surface area) for women in predicting rAAA.31 The ASI for women is higher than that of men for both intact and rupture AAA repair. Therefore, the discussion regarding thresholds for elective repair in women should be expanded to use a more appropriate metric. Women have a significantly higher mortality than men following elective AAA repair and are less likely to be eligible for EVAR.32-34 Due to lack of data, it is not possible to clearly determine the risk of rAAA in women stratified by AAA diameter or ASI and so further studies are required to inform future practice.35 Similarly, current thresholds may not be appropriate for ethnic minorities who experience increased incidence of perioperative and postoperative complications.36,37 It is unclear whether this is due to socioeconomic factors such as access to high volume vascular centres or genetic factors that could account for increased incidence of comorbidities. Nonetheless, recognising these differences would include considering increasing the threshold of elective AAA repair in these demographics.
Personalised thresholds
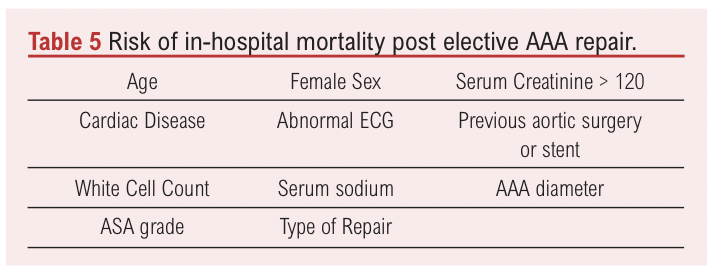
A step towards personalised medicine in AAA has included the British Aneurysm Repair (BAR) Score, a multivariate model for elective AAA repair by OSR or EVAR (Table 5).38 It provides an estimate of the risk of in-hospital mortality based on data from the National Vascular Database. The Aneurysm Repair Decision Aid (ARDA) is another tool that uses patient data from the RESCAN project with an aim to inform if elective AAA repair is optimal for an individual patient.29 It takes into consideration factors such as advancing age, AAA growth and comorbidities. The broad recommendations of this tool suggest earlier repair in younger and fitter patients and in contrast surveillance in elderly patients with comorbidities. These multifactorial tools, if validated, will likely replace individual scores such as the AAA diameter for determining if elective AAA repair is appropriate.
Conclusions
The elective threshold would be best considered on a case-by-case basis. Population-based analysis has provided strong recommendations but widespread applicability is questionable. Furthermore, the changing landscape of AAA incidence and repair means the conclusions of older studies must be reconsidered. The use of a more sophisticated scoring system will likely replace the single metric of AAA diameter but in the interim it is unlikely the threshold will be increased to as high as 7cm from 5.5cm but trials should be carried out to evaluate alternative thresholds.
References
1. Verhagen H, Guidelines Committee E. Editor’s choice–European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. European Journal of [Internet]. 2019; Available from: https://www.ejves.com/article/S1078-5884(18)30698-1/abstract
2. Brown LC, Thompson SG, Greenhalgh RM, Powell JT, UK Small Aneurysm Trial Participants. Fit patients with small abdominal aortic aneurysms (AAAs) do not benefit from early intervention. J Vasc Surg 2008;48(6):1375–81. https://doi.org/10.1016/j.jvs.2008.07.014
3. Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group.
Ann Intern Med 1997;126(6):441–9.
https://doi.org/10.7326/0003-4819-126-6-199703150-00004
4. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg 2011;41(1):13–25. https://doi.org/10.1016/j.ejvs.2010.08.026
5. Ouriel K. The PIVOTAL study: a randomized comparison of endovascular repair versus surveillance in patients with smaller abdominal aortic aneurysms. J Vasc Surg 2009;49(1):266–9. https://doi.org/10.1016/j.jvs.2008.11.048
6. Lederle FA, Johnson GR, Wilson SE, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002; 287(22):2968–72. https://doi.org/10.1001/jama.287.22.2968
7. Parkinson F, Ferguson S, Lewis P, Williams IM, Twine CP, South East Wales Vascular Network. Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. J Vasc Surg 2015;61(6): 1606–12. https://doi.org/10.1016/j.jvs.2014.10.023
8. Buth J. Endovascular repair of abdominal aortic aneurysms. Results from the EUROSTAR registry. EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair. Semin Interv Cardiol 2000;5(1):29–33.
9. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg 2001;33(4):752–7. https://doi.org/10.1067/mva.2001.112800
10. Sahal M, Prusa AM, Wibmer A, et al. Elective abdominal aortic aneurysm repair: does the aneurysm diameter influence long-term survival? Eur J Vasc Endovasc Surg 2008;35(3):288–94. https://doi.org/10.1016/j.ejvs.2007.09.019
11. De Rango P, Cao P, Parlani G, Verzini F, Brambilla D. Outcome after endografting in small and large abdominal aortic aneurysms: a metanalysis. Eur J Vasc Endovasc Surg 2008;35(2):162–72. https://doi.org/10.1016/j.ejvs.2007.10.015
12. de Guerre LEVM, Dansey K, Li C, et al. Late outcomes after endovascular and open repair of large abdominal aortic aneurysms. J Vasc Surg 2021;74(4): 1152–60. https://doi.org/10.1016/j.jvs.2021.02.024
13. Keith CJ Jr, Passman MA, Gaffud MJ, et al. Comparison of outcomes following endovascular repair of abdominal aortic aneurysms based on size threshold. J Vasc Surg 2013;58(6):1458–66. https://doi.org/10.1016/j.jvs.2013.06.060
14. Huang Y, Gloviczki P, Duncan AA, et al. Maximal aortic diameter affects outcome after endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2017;65(5):1313–22.e4. https://doi.org/10.1016/j.jvs.2016.10.093
15. Brahmbhatt R, Gander J, Duwayri Y, et al. Improved trends in patient survival and decreased major complications after emergency ruptured abdominal aortic aneurysm repair. J Vasc Surg 2016;63(1):39–47. https://doi.org/10.1016/j.jvs.2015.08.050
16. Bastos Gonçalves F, Ultee KHJ, Hoeks SE, Stolker RJ, Verhagen HJM. Life expectancy and causes of death after repair of intact and ruptured abdominal aortic aneurysms. J Vasc Surg 2016;63(3):610–16. https://doi.org/10.1016/j.jvs.2015.09.030
17. Laine MT, Laukontaus SJ, Sund R, et al. A Population-Based Study of Abdominal Aortic Aneurysm Treatment in Finland 2000 to 2014. Circulation 2017;136(18):1726–34. https://doi.org/10.1161/CIRCULATIONAHA.117.028259
18. De Martino RR, Eldrup-Jorgensen J, Nolan BW, et al. Perioperative management with antiplatelet and statin medication is associated with reduced mortality following vascular surgery. J Vasc Surg 2014;59(6):1615–21, 1621.e1. https://doi.org/10.1016/j.jvs.2013.12.013
19. Kertai MD, Boersma E, Westerhout CM, et al. Reprinted article “A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.” Eur J Vasc Endovasc Surg 2011;42 Suppl 1:S96–104. https://doi.org/10.1016/j.ejvs.2011.06.016
20. McNally MM, Agle SC, Parker FM, Bogey WM, Powell CS, Stoner MC. Preoperative statin therapy is associated with improved outcomes and resource utilization in patients undergoing aortic aneurysm repair. J Vasc Surg 2010;51(6):1390–6. https://doi.org/10.1016/j.jvs.2010.01.028
21. Yoshimura K, Morikage N, Nishino-Fujimoto S, Furutani A, Shirasawa B, Hamano K. Current Status and Perspectives on Pharmacologic Therapy for Abdominal Aortic Aneurysm. Curr Drug Targets 2018;19(11):1265–75. https://doi.org/10.2174/1389450119666171227223331
22. Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RAP. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg 2007;94(6):696–701. https://doi.org/10.1002/bjs.5780
23. Wanhainen A, Hultgren R, Linné A, et al. Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation 2016;134(16): 1141–8. https://doi.org/10.1161/CIRCULATIONAHA.116.022305
24. Guirguis-Blake JM, Beil TL, Senger CA, Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160(5):321–9. https://doi.org/10.7326/M13-1844
25. Lederle FA. The Last (Randomized) Word on Screening for Abdominal Aortic Aneurysms. JAMA Intern Med 2016;176(12):1767–8. https://doi.org/10.1001/jamainternmed.2016.6663
26. Glover MJ, Kim LG, Sweeting MJ, Thompson SG, Buxton MJ. Cost-effectiveness of the National Health Service Abdominal Aortic Aneurysm Screening Programme in England. Br J Surg 2014;101(8):976–82. https://doi.org/10.1002/bjs.9528
27. McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26(9):733–44. https://doi.org/10.2165/00019053-200826090-00004
28. Sweeting MJ, Marshall J, Glover M, Nasim A, Bown MJ. Evaluating the Cost-Effectiveness of Changes to the Surveillance Intervals in the UK Abdominal Aortic Aneurysm Screening Programme. Value Health 2021;24(3):369–76. https://doi.org/10.1016/j.jval.2020.10.015
29. RESCAN Collaborators, Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA 2013;309(8):806–13. https://doi.org/10.1001/jama.2013.950
30. United Kingdom Small Aneurysm Trial Participants, Powell JT, Brady AR, Brown LC, et al. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002; 346(19):1445–52. https://doi.org/10.1056/NEJMoa013527
31. Lo RC, Lu B, Fokkema MTM, et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg 2014;59(5):1209–16. https://doi.org/10.1016/j.jvs.2013.10.104
32. Ulug P, Sweeting MJ, von Allmen RS, et al. Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet 2017;389(10088):2482–91. https://doi.org/10.1016/S0140-6736(17)30639-6
33. Grootenboer N, van Sambeek MRHM, Arends LR, Hendriks JM, Hunink MGM, Bosch JL. Systematic review and meta-analysis of sex differences in outcome after intervention for abdominal aortic aneurysm. Br J Surg 2010;97(8): 1169–79. https://doi.org/10.1002/bjs.7134
34. Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011; 123(24):2848–55. https://doi.org/10.1161/CIRCULATIONAHA.110.014902
35. Vavra AK, Kibbe MR, Bown MJ, Powell JT. Debate: Whether evidence supports reducing the threshold diameter to 5 cm for elective interventions in women with abdominal aortic aneurysms. J Vasc Surg 2014;60(6):1695–701. https://doi.org/10.1016/j.jvs.2014.07.022
36. Deery SE, O’Donnell TFX, Shean KE, et al. Racial disparities in outcomes after intact abdominal aortic aneurysm repair. J Vasc Surg 2018;67(4):1059–67. https://doi.org/10.1016/j.jvs.2017.07.138
37. Williams TK, Schneider EB, Black JH 3rd, et al. Disparities in outcomes for Hispanic patients undergoing endovascular and open abdominal aortic aneurysm repair. Ann Vasc Surg 2013;27(1):29–37. https://doi.org/10.1016/j.avsg.2012.06.006
38. Grant SW, Hickey GL, Grayson AD, Mitchell DC, McCollum CN. National risk prediction model for elective abdominal aortic aneurysm repair. Br J Surg 2013; 100(5):645–53. https://doi.org/10.1002/bjs.9047
39. Grant SW, Sperrin M, Carlson E, et al. Calculating when elective abdominal aortic aneurysm repair improves survival for individual patients: development of the Aneurysm Repair Decision Aid and economic evaluation. Health Technol Assess 2015;19(32):1–154, v–vi. https://doi.org/10.3310/hta19320
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(4):250-256
Publication date:
August 31, 2024











