WINNING ESSAYS
Rouleaux Club Winning Essays 2023
The Rouleaux Club run an annual essay competition to help promote interest in vascular surgery. Entrants are asked to write 1,500 words on one of three topics selected by the RC Executive. The essays are marked by the committee and the prizes are awarded to the best essay at the annual Vascular Society meeting. There are two prize categories, one for medical students and another for junior doctors. Following the Vascular Societies GB&I Annual Scientific Meeting, the winning essays will be published in the journal.
STUDENT CATEGORY
Is surgical bypass underutilised for patients requiring lower limb revascularisation?
Luke Davies, University of Bristol
Introduction
For decades, surgical bypass provided the mainstay of treatment for the most advanced presentation of peripheral arterial disease (PAD), now referred to as chronic limb-threatening ischaemia (CLTI).1-3 However, the introduction and technical advancements of less-invasive endovascular approaches has led to an undeniable global decline over the past 10-20 years, as demonstrated in Figure 1.4-7
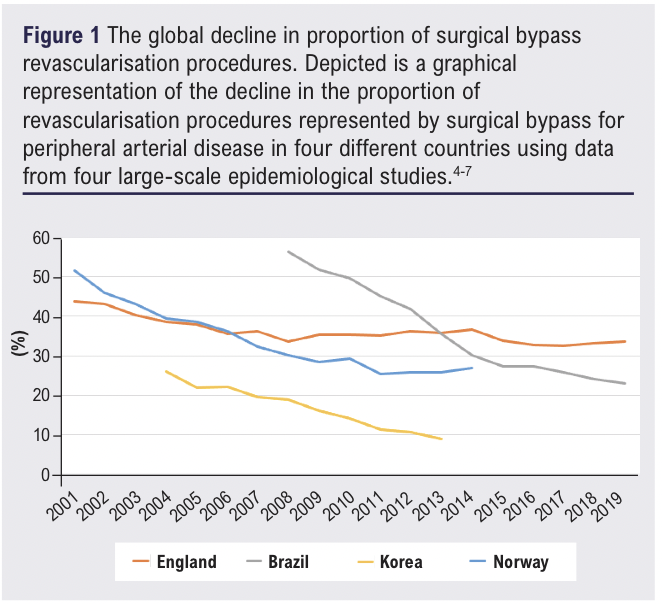
Due to a widely acknowledged paucity of high-quality evidence, guidelines remain inconsistent and unclear with their recommendations. Given that CLTI affects 11% of the estimated 230 million living with PAD,8-10 and the constant strive to minimise procedural and disease-related complications, it is important to question whether the above decline is justified. Following critical evaluation of the evidence and current guidelines, it will be argued that the decline in rates of surgical bypass is justified by the growing body of evidence to suggest endovascular revascularisation as an adequate, less-invasive and lower-cost alternative.
Reviewing the evidence
Only three large-scale randomised-control trials (RCTs) comparing patient outcomes following endovascular and surgical revascularisation for CLTI have been published, and provide a seemingly conflicting range of results. It is important that results are not taken solely at face-value, and are instead interpreted in context through critical appraisal.
The demand for an RCT comparing the two techniques was first met by the BASIL-1 trial in 2005.11 452 patients with severe limb ischaemia (synonymous with CLTI) due to infra-inguinal disease were randomised to receive a surgery-first or angioplasty-first approach. At 2 years, there was no significant difference in amputation-free survival (the primary outcome). However, it is important to question the relevance of these results to modern clinical practice, given that endovascular techniques have advanced rapidly since the completion of this trial. There is growing evidence to support a plethora of novel techniques, including stenting, drug-eluting technology and atherectomy.12,13 The endovascular-first strategy was limited mostly to angioplasty alone, with stent-usage largely excluded. Thus, it no longer represents the ideal endovascular strategy for patients with CLTI. Given the rapid technical advancements of endovascular procedures, it is not unsurprising that technical failure rates have substantially decreased since this trial.14-16 Furthermore, the trial was not specific regarding anatomical complexity, making it difficult to apply the results to a clinical environment in which decisions are frequently made with this in mind. Finally, in the surgical group, both autologous and prosthetic grafts were employed, with the latter now considered inferior.17 Therefore, an ideal surgical strategy is also not represented.
The long-awaited BEST-CLI trial18 sought to address these limitations by investigating a greater number of patients, with emphasis on availability of autologous vein graft. Two parallel cohorts were investigated. Cohort 1 (n=1,434) included patients with CLTI and an identified adequate great saphenous vein, while cohort 2 (n=396) included those without. Patients in each were randomised to undergo either surgical or endovascular revascularisation. The primary outcome was a composite of major adverse limb events (MALE) or death, which occurred significantly less in the surgical group in cohort 1. In contrast, the incidence of the primary outcome in cohort 2 was comparable between the two groups. While these findings seem to suggest a bypass-first approach be optimal for those with an adequate great-saphenous vein, it has not been without criticism.19,20 Despite taking place roughly 15 years after the BASIL trial, the technical failure of endovascular therapy in cohort 1 was 15%, a figure similar to that of the BASIL trial, and higher than reported in contemporary data.15-17 The primary composite outcome was mainly driven by reintervention rates, with no significant difference between the two groups in cohort 1 with regards to death or above-ankle amputation. Given the high percentage of technical failure in the endovascular group, it is unsurprising that 42.5% of first reinterventions in this group occurred within the first 30 days. Furthermore, there was a large degree of heterogeneity in endovascular procedure techniques. It appears the single best surgical-first intervention has not been compared to the single best endovascular-first treatment available at the time; results should be interpreted with this in mind. Furthermore, data regarding the anatomical complexity of disease is yet to be published, again making application to real-world clinical scenarios challenging.
The results of the BEST-CLI trial are seemingly contradicted by the findings of the BASIL-2 trial21 published in 2023. This was the first to find improved outcomes with endovascular intervention compared to bypass in the context of CLTI. BASIL-2 specifically investigated those requiring infra-popliteal revascularisation, which made up 55% of revascularisation procedures included in the BEST-CLI trial. All 345 patients were randomised to receive either endovascular-first or vein-bypass-first treatment, with the primary outcome (amputation-free survival) significantly higher in the endovascular group. Despite the surgical group being described as “vein-bypass-first”, availability of an adequate great saphenous vein was not part of the eligibility criteria. Vein usage was up to the discretion of the surgeon, with the use of prosthetic grafts permitted, a contradiction to the description of the group and the trial name. Nevertheless, the BASIL-2 trial further demonstrates that endovascular revascularisation can be a suitable, less-invasive alternative to surgical bypass.
Table 1 summarises the findings and limitations of the RCTs published comparing endovascular revascularisation and surgical bypass for those with CLTI. These trials appear to seek a black and white answer to a question which deserves a multi-faceted approach. By doing so, there is a constant lack of appreciation for the important details. Anatomical complexity is often disregarded or an afterthought, and there is consistent neglect for either the “ideal” endovascular strategy or “ideal” bypass graft in all trials. This makes application to real-world scenarios challenging. However, when limitations are accounted for there appears to be a case for endovascular revascularisation as an adequate alternative to surgical bypass, particularly with rapidly improving endovascular techniques and falling associated technical failure. Given that endovascular revascularisation can offer comparable outcomes to bypass in patients with a range of disease-complexity, it is important to critically review current guidelines to ascertain whether they provide a robust foundation for clinical decision making. This evaluation will determine whether circumstance-specific decisions represent a justified growing preference for endovascular revascularisation.
What are the current guidelines?
Current guidelines are relatively vague, owing to the established limited body of evidence. The most detailed and circumstance-specific guidelines for CLTI decision-making were created in 2019, known as the Global Vascular Guidelines (GVG).22 It is firstly recommended that any inflow disease, defined as proximal to the origin of the profunda femoris be treated prior to outflow disease. The guidelines then provide a systematic approach, termed Evidence-Based Revascularisation, to decide between an endovascular-first or bypass-first strategy for remaining patients. This is demonstrated by the decision-making tool shown in Figure 2. This approach is more specific than previous guidelines, and results in a vastly smaller proportion of patients deemed as best suited for a bypass-first approach.23-25
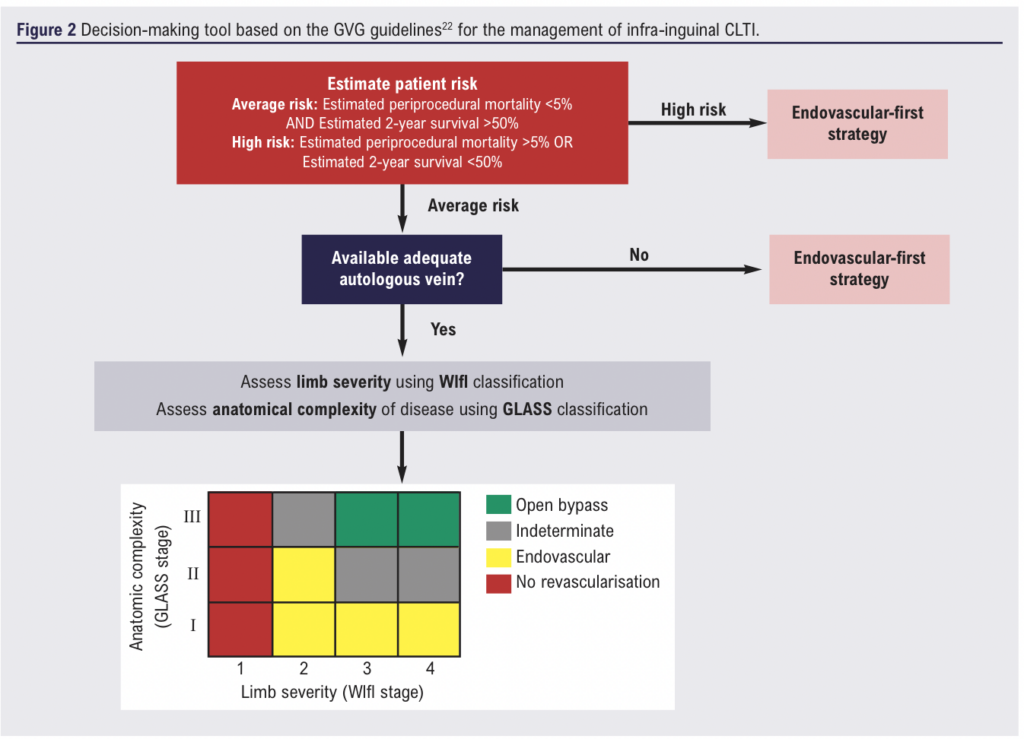
While these circumstance-specific guidelines are the most current, they were published prior to publication of the BEST-CLI and BASIL-2 trials. They were fuelled by a variety of prospective and retrospective studies, rather than any of the large RCTs, hence the low level of evidence (level C). Furthermore, the paucity of evidence to guide the WIfI and GLASS based recommendations leave the approach somewhat flawed. Firstly, there exists a large indeterminate range in which no optimal revascularisation approach is recommended, as shown in Figure 2. Additionally, the GLASS staging system is new, and various flaws in its usage have been identified. This includes low inter-observer agreement among clinicians, as well as an inability to predict patency rates following endovascular revascularisation in those with lower stages.26,27 These flaws merit a holistic approach to decision making, considering a range of different factors, rather than relying entirely on guidelines. This includes patient preferences which will tend to favour the less-invasive endovascular option, costs which again tend to favour endovascular therapy (although long-term cost-effectiveness is still unclear), and the clinician’s experience.28-30
Conclusion – Is the decline justified?
There is a growing high-quality body of evidence to justify endovascular revascularisation as an alternative to bypass in a range of patients with varying disease-patterns and severities of CLTI. The low class of evidence underpinning the current guidelines warrants the adoption of a decision-making approach informed by a range of important different factors, particularly in the indeterminate group. Costs and patient preference will play a large role in these decisions, and both will likely favour endovascular therapy over bypass. Therefore, despite the global decline in use of surgical bypass, it is not yet an under-utilised entity in the management of CLTI.
Nevertheless, surgical bypass will remain a crucial management option, particularly following failed endovascular therapy and in those with well-established anatomically complex disease. Thus, in contrast to current attitudes, surgical bypass and endovascular revascularisation should be viewed as complementary techniques with the combined aim of preserving patient function and quality of life, rather than continue to be pitted against one another. Such an outlook, paired with an improved regard for disease and procedural specificity in future trials, will ensure neither option goes under-utilised in the future.
References
1. Treiman GS, Treiman RL, Ichikawa L, Van Allan R. Should percutaneous transluminal angioplasty be recommended for treatment of infrageniculate popliteal artery or tibioperoneal trunk stenosis? J Vasc Surg 1995;22(4): 457-65. https://doi.org/10.1016/S0741-5214(95)70015-3
2. Blair JM, Gewertz BL, Moosa H, Lu CT, Zarins CK. Percutaneous transluminal angioplasty versus surgery for limb-threatening ischemia. J Vasc Surg 1989; 9(5):698-703. https://doi.org/10.1016/S0741-5214(89)70042-2
3. Berchiolli R, Bertagna G, Adami D, Canovaro F, Torri L, Troisi N. Chronic Limb-Threatening Ischemia and the Need for Revascularization. J Clin Med 2023; 12(7):2682. https://doi.org/10.3390/jcm12072682
4. Staniszewska A, Gimzewska M, Onida S, Lane T, Davies AH. Lower extremity arterial interventions in England. Ann R Coll Surg Engl 2021;103(5):360-6. https://doi.org/10.1308/rcsann.2020.7090
5. Wolosker N, Silva MF, Portugal MF, et al. Epidemiological analysis of lower limb revascularization for peripheral arterial disease over 12 years on the public healthcare system in Brazil. J Vasc Bras 2022;21:e20210215. https://doi.org/10.1590/1677-5449.202102152
6. Park YY, Joh JH, Han SA, et al. National trends for the treatment of peripheral arterial disease in Korea between 2004 and 2013. Ann Surg Treat Res 2015;89(6):319-24. https://doi.org/10.4174/astr.2015.89.6.319
7. Wendt K, Kristiansen R, Krohg-Sørensen K, Gregersen FA, Fosse E. Norwegian trends in numbers of lower extremity revascularisations and amputations including regional trends in endovascular treatments for peripheral arterial disease: a retrospective cross-sectional registry study from 2001 to 2014. BMJ open 2017;7(11):e016210. https://doi.org/10.1136/bmjopen-2017-016210
8. Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. The Lancet Global Health 2019;7(8):e1020-30. https://doi.org/10.1016/S2214-109X(19)30255-4
9. Eid MA, Mehta KS, Goodney PP. Epidemiology of peripheral artery disease. In Seminars in Vascular Surgery 2021;34(1):38-46. WB Saunders. https://doi.org/10.1053/j.semvascsurg.2021.02.005
10. Kwong M, Rajasekar G, Utter GH, Nuño M, Mell MW. Updated estimates for the burden of chronic limb-threatening ischemia in the Medicare population. J Vasc Surg 2023;77(6):1760-75. https://doi.org/10.1016/j.jvs.2023.01.200
11. Bradbury AW, Ruckley CV, Fowkes FG, Forbes JF, Gillespie I, Adam DJ. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366(9501):1925-34. https://doi.org/10.1016/S0140-6736(05)67704-5
12. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circulation: Cardiovascular Interventions 2011;4(5):495-504. https://doi.org/10.1161/CIRCINTERVENTIONS.111.962324
13. Beckman JA, Schneider PA, Conte MS. Advances in revascularization for peripheral artery disease: revascularization in PAD. Circulation Research 2021;128(12):1885-912. https://doi.org/10.1161/CIRCRESAHA.121.318261
14. Iida O, Takahara M, Soga Y, Kodama A, Terashi H, Azuma N. Three-year outcomes of surgical versus endovascular revascularization for critical limb ischemia: the SPINACH study (surgical reconstruction versus peripheral intervention in patients with critical limb ischemia). Circulation: Cardiovascular Interventions 2017;10(12):e005531. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005531
15. Lee RE, Patel A, Soon SX, Chan SL, Yap CJ, Chandramohan S, Tay LH, Chong TT, Tang TY. One year clinical outcomes of Rutherford 6 chronic limb threatening ischemia patients undergoing lower limb endovascular revascularisation from Singapore. CVIR Endovascular 2022;5(1):1-2. https://doi.org/10.1186/s42155-022-00306-1
16. Giannopoulos S, Palena LM, Armstrong EJ. Technical success and complication rates of retrograde arterial access for endovascular therapy for critical limb ischaemia: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2021;61(2):270-9. https://doi.org/10.1016/j.ejvs.2020.11.020
17. Ambler GK, Twine CP. Graft type for femoro‐popliteal bypass surgery. Cochrane Database of Systematic Reviews. 2018(2). https://doi.org/10.1002/14651858.CD001487.pub3
18. Farber A, Menard MT, Conte MS, et al. Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med 2022;387(25):2305-16. https://doi.org/10.1056/NEJMoa2207899
19. Takahashi EA, Lookstein RA, Misra S. Best Endovascular versus Best Surgical Therapy in Patients with CLI (BEST-CLI) Trial: A Misleading Trial Name. J Vasc Interv Radiol 2023;34(4):718-19. https://doi.org/10.1016/j.jvir.2023.01.005
20. Cormican DS, Healy DA, Chess BA. Surgical revascularization versus endovascular therapy to treat chronic limb-threatening ischemia: perhaps less invasive is not always better. J Cardiothorac Vasc Anesth 2023;37(7):1072-4. https://doi.org/10.1053/j.jvca.2023.03.026
21. Bradbury AW, Moakes CA, Popplewell M, et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. The Lancet 2023;401(10390):1798-809. https://doi.org/10.1016/S0140-6736(23)00462-2
22. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58(1):S1-109. https://doi.org/10.1016/j.ejvs.2019.05.006.
23. Gerhard-Herman MD, Gornik HL, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;69(11): e71-126.
24. Aboyans V, Ricco JB, Bartelink ML, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Kardiologia Polska (Polish Heart Journal) 2017;75(11):1065-160. https://doi.org/10.5603/KP.2017.0216
25. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Tasc II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45(1):S5-67. https://doi.org/10.1016/j.jvs.2006.12.037
26. Wijnand JG, Zarkowsky D, Wu B, et al. The global limb anatomic staging system (GLASS) for CLTI: improving inter-observer agreement. J Clin Med 2021;10(16):3454. https://doi.org/10.3390/jcm10163454
27. Bontinis V, Bontinis A, Koutsoumpelis A, Giannopoulos A, Ktenidis K. A systematic review and meta-analysis of GLASS staging system in the endovascular treatment of chronic limb threatening ischemia. J Vasc Surg 2022 Aug 8. https://doi.org/10.1016/j.jvs.2022.07.183
28. Vossen RJ, Philipszoon PC, Vahl AC, Montauban van Swijndregt AD, Leijdekkers VJ, Balm R. A comparative cost-effectiveness analysis of percutaneous transluminal angioplasty with optional stenting and femoropopliteal bypass surgery for medium-length TASC II B and C femoropopliteal lesions. J Endovasc Ther 2019;26(2):172-80. https://doi.org/10.1177/1526602819833646
29. Perlander A, Broeren M, Österberg K, Svensson M, Nordanstig J. Disease specific health related quality of life in patients with chronic limb threatening ischaemia undergoing revascularisation of femoropopliteal lesions. Eur J Vasc Endovasc Sur 2023;66(2):245-51. https://doi.org/10.1016/j.ejvs.2023.05.014
30. Forbes JF, Adam DJ, Bell J, et al., trial Participants BA. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010;51(5):43S-51S. https://doi.org/10.1016/j.jvs.2010.01.076
DOCTOR CATEGORY
Discuss the role of Artificial Intelligence (AI) in improving current management of Abdominal Aortic Aneurysms (AAA)
Ishtar Redman, St George’s University Hospitals NHS Foundation Trust
Introduction
Abdominal aortic aneurysmal disease encompasses a diverse spectrum of pathologies and clinical presentations ranging from incidental findings on imaging to a patient presenting in extremis following rupture. The management of this diverse patient cohort depends largely on clinical presentation and patient characteristics. Artificial intelligence (AI) has permeated modern medicine at an unprecedented rate. In the healthcare landscape, the primary utility of AI lies in clinically tailored neural networks and machine learning. In the field of Vascular Surgery, several AI technologies have been, or are currently being developed in relation to pre-operative planning, improving intra-operative efficiency and assessing post-operative outcomes for patients with abdominal aortic aneurysms (AAA). Despite the exponential development of these technologies, they are not without their limitations, and the current National Health Service is far from implementing them into routine clinical practice.
Literature review
Electronic searches were performed on both Medline (1946 to August 2023) and Embase (1974 to August 2023) using the OVID interface as well as Medline using the PubMed interface. The search terms were as follows: (artificial intelligence OR AI OR machine learning OR neural network*) AND (abdominal aortic aneurysm* OR AAA) AND (management OR treatment). These keywords were searched in the subject headings, in title and in abstract. All reference lists of the included papers were also screened to identify any pertinent studies. The results were current as of August 2023.
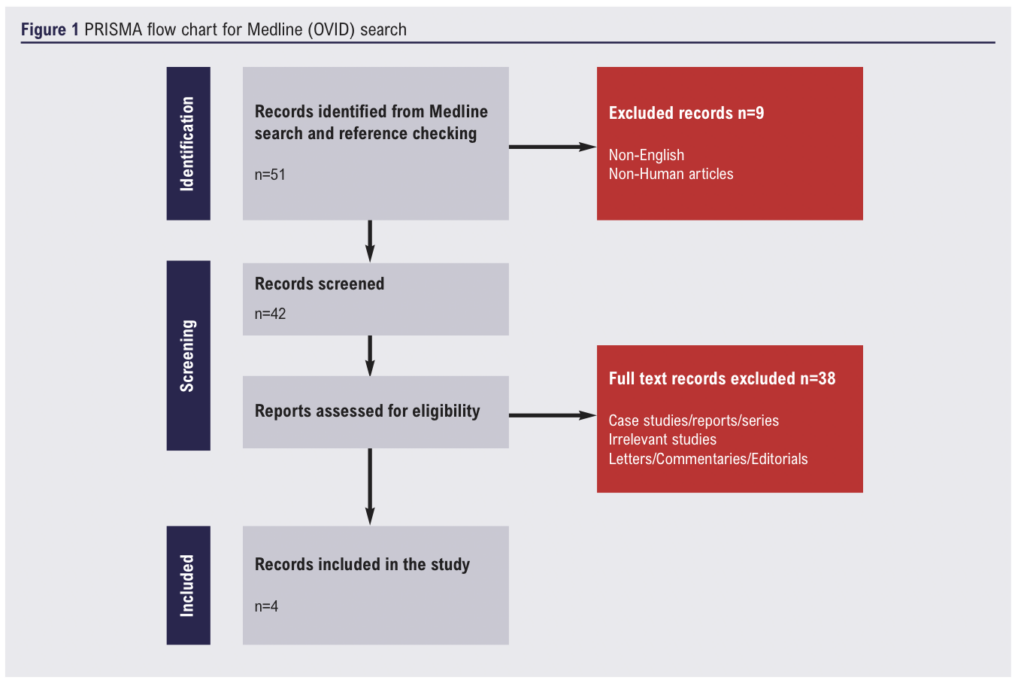
732 papers were found using the reported search on Embase (61) and Medline (51) using the Ovid interface whilst 620 were found on Medline using the PubMed interface. Case reports, case studies, editorials, duplicates, and literature reviews were excluded. An example of the screening and eligibility assessment process for the search results obtained from the Medline (Ovid) interface is outlined in the PRISMA diagram below (Figure 1).
Prediction models, image segmentation and automation
In the United Kingdom, guidelines from the Society for Vascular Surgery and the European Society for Vascular Surgery1,2 have clear recommendations for the diagnosis and management of patients with AAA. Decisions regarding treatment rely on careful evaluation of the risks associated with operative intervention compared to the risk of aneurysmal growth and rupture. Although initial aneurysmal diameter is a well-established, independent risk factor for rupture, other patient and aneurysm specific factors have been implicated.3 In practice, estimating sac progression and predicting the risk of rupture can be difficult for clinicians, consequently, AI prediction and prognostication models have been developed to assist vascular surgeons with this undertaking. The maximal aortic diameter is often used in this decision-making process, however, a major downfall of utilising this single measurement, lies in its inability to provide details of the three dimensional (3D) volumetric evaluation of the aneurysm sac. To acquire such characteristics, manual image analysis would need to be undertaken, a laborious and specialist endeavour. Furthermore, in even the most experienced hands, significant discrepancies have been identified, with one study reporting up to 87% of diameter measurements falling outside of the clinically acceptable error range (± 5 mm) in abdominal aortic aneurysms (AAAs).4 Specific deep learning workstations have been designed to perform analysis of images generated from CT-Aortograms, allowing for more accurate three-dimensional (3D) evaluation of the complete aortic anatomy. The advantages of acquiring volumetric data for these aneurysms cannot be understated and one can appreciate how this type of analysis can improve the sensitivity of estimating disease progression compared to maximum diameter measurements taken in isolation. AI programs have been designed to perform large scale quantitative analysis of AAAs with improved image segmentation by characterising aneurysmal morphology, geometry, and fluid dynamics.5 This data is then fed into computational AI models with predictive and prognostic capabilities, allowing for recognition of patterns which in turn, can be used to estimate the rates of AAA growth and risk of rupture. PRAEVAorta (Nurea) is a ‘decision support AI software’ designed by a French company with prediction abilities to describe the evolution of AAA based on geometric and flow characteristics.6 The same company is currently in the research phase of developing technology which allows for automated analysis of AAA sac measurements pre and post-EVAR.
Pre-operative planning and intra-operative uses
Move over SHO, AI is scrubbing in. Artificial intelligence has found its way into the operating theatre with complex machine learning models assisting with preoperative surgical and endovascular planning as well as on-table image guidance.7 These algorithms have been developed to optimise stent or device selection, endovascular navigation, and stent placement. Cydar Medical, in conjunction with researchers at Kings College London, are utilising a similar type of vascular navigation technology (Cydar EV Maps) in the ARIA trial – ARtificially Intelligent image fusion system in comparison to standard treatment to guide endovascular Aortic aneurysm repair.8,9 The trial is currently in the recruitment phase. In a similar vein, AI protocols utilising electromagnetic tracking technology have been developed with the aim of improving surgical efficiency by reducing procedural time and decreasing patient and operator radiation exposure. The Intraoperative Positioning System (IOPS) (Centerline Biomedical) is a 3D image guidance system which employs structural mapping and electromagnetic tracking technology embedded into operating kit (catheters, guidewires) to reduce on-table radiation exposure.7 Risk assessment and prognostication programs, like those detailed for image segmentation above, were also developed to assess postoperative outcomes, including mortality and potential complications after endovascular repair.10,11
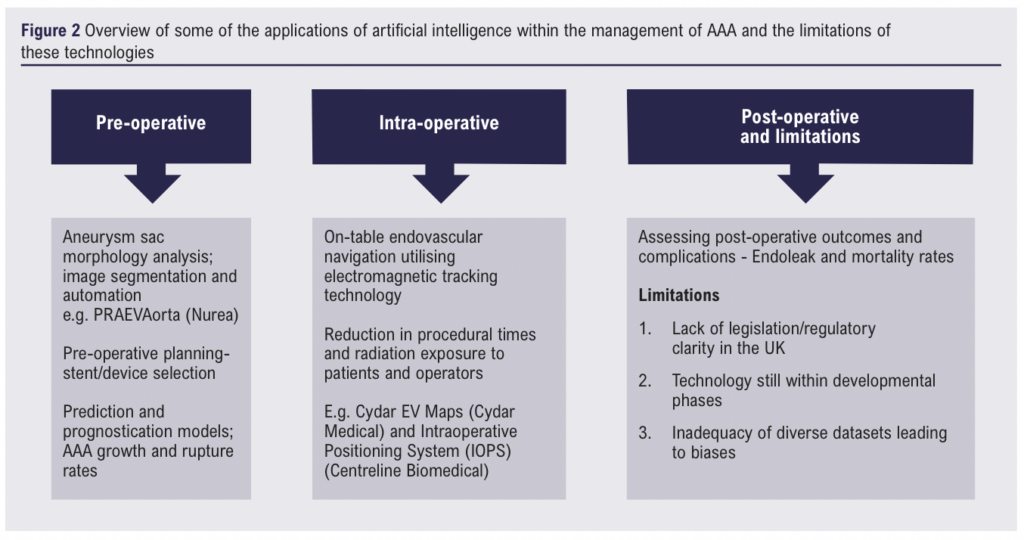
Challenges, limitations, and bias
Most artificial intelligence algorithms require high-quality and diverse databases to ensure accuracy and robustness when translated to real-life settings.12 One major issue ubiquitous within the fields of AI and machine learning, is the acquisition of such high-quality datasets allowing for training and evaluating ML algorithms. Furthermore, there is often a lack of standardisation when curating the imaging databases used in AI associated vascular surgery algorithms, making it difficult to compare and interpret results across different studies.13 Another major barrier impeding the uptake of AI in healthcare is the lack of clarity regarding legislation, data security and regulation. In the United States for instance, the Food and Drug Administration (FDA) is responsible for approving and regulating AI and ML tools, however in the UK, explicit regulatory frameworks and legislation do not yet exist. Instead, AI’s use within healthcare is currently regulated under fragments of pre-existing general legislation, such as the UK Medical Device Regulations 2002 or the Data Protection Act 2018.14 This ambiguity can complicate and impede the scientific process and may even lead to frivolous litigation, for example, the class-action lawsuit levied at Google’s DeepMind AI, following the patient data scandal at the Royal Free in 2016.15
Interestingly, the concept of bias within AI and ML algorithms has become somewhat of an area of controversy. The potential sources for these biases include inherently biased software or program designs and incorrect or unbalanced training data being fed into the algorithms.16 This is especially important when applying algorithms within vascular surgery, where the patient cohort is increasingly diverse and management and post-operative outcomes are dependent on patient variables such as sex and race.17 One potential means of mitigating these biases would be the inclusion of large, diverse datasets and proactive algorithmic testing for biases within experimental stages of technological development.
Conclusions
Artificial intelligence has made substantial advancements in the field of vascular surgery and has the potential to revolutionise the management of patients with abdominal aortic aneurysms by facilitating earlier detection, improving the accuracy of measurements, personalising treatment plans and optimising operative proceedings. The applications of AI detailed within this piece are by no means exhaustive, and progress within this area is occurring at an unprecedented rate. Despite this, there are many challenges and limitations to the development, commercialisation, and uptake of AI within everyday clinical practice.
References
1. Gloviczki P, Lawrence PF, Forbes TL. Update of the Society for Vascular Surgery Abdominal Aortic Aneurysm Guidelines. J Vasc Surg 2018;67(1):1. https://doi.org/10.1016/j.jvs.2017.11.022
2. Dalman RL. The 2019 update of the European Abdominal Aortic Aneurysm Guidelines. J Vasc Surg 2019;69(3):633–4. https://doi.org/10.1016/j.jvs.2018.12.008
3. Kessler V, Klopf J, Eilenberg W, Neumayer C, Brostjan C. AAA revisited: A comprehensive review of risk factors, management, and hallmarks of pathogenesis. Biomedicines 2022;10(1):94.
https://doi.org/10.3390/biomedicines10010094
4. Mora C, Marcus C, Barbe C, Ecarnot F, Long A. Measurement of maximum diameter of native abdominal aortic aneurysm by Angio-CT: Reproducibility is better with the semi-automated method. Eur J Vasc Endovasc Surg 2014; 47(2):139–50. https://doi.org/10.1016/j.ejvs.2013.10.013
5. Raffort J, Adam C, Carrier M, et al. Artificial Intelligence in abdominal aortic aneurysm. J Vasc Surg 2020;72(1). https://doi.org/10.1016/j.jvs.2019.12.026
6. Caradu C, Spampinato B, Vrancianu AM, Bérard X, Ducasse E. Fully automatic volume segmentation of infrarenal abdominal aortic aneurysm computed tomography images with deep learning approaches versus physician controlled manual segmentation. J Vasc Surg 2021;74(1). https://doi.org/10.1016/j.jvs.2020.11.036
7. Stonko DP, Morrison JJ, Hicks CW. A review of mature machine learning- and artificial intelligence-enabled applications in aortic surgery. JVS-Vascular Insights 2023;1:100016. https://doi.org/10.1016/j.jvsvi.2023.100016
8. Patel RJ, Lee AM, Hallsten J, Lane JS, Barleben AR, Malas MB. Use of surgical augmented intelligence maps can reduce radiation and improve safety in the endovascular treatment of complex aortic aneurysms. J Vasc Surg 2023;77(4):982-990.e2. https://doi.org/10.1016/j.jvs.2022.12.033
9. Cydar Medical and King’s college London initiate Aria Trial of Cydar EV Maps System [Internet]. Bryn Mawr Communications; 2022 [cited 2023 Aug 31]. Available from: https://evtoday.com/news/cydar-medical-and-kings-college-london-initiate-aria-trial-of-cydar-ev-maps-system
10. Wise ES, Hocking KM, Brophy CM. Prediction of in-hospital mortality after ruptured abdominal aortic aneurysm repair using an artificial neural network. J Vasc Surg 2015;62(1):8–15. https://doi.org/10.1016/j.jvs.2015.02.038
11. Li B, Feridooni T, Cuen-Ojeda C, et al. Machine learning in vascular surgery: A systematic review and critical appraisal. npj Digital Medicine 2022;5(1). https://doi.org/10.1038/s41746-021-00552-y
12. Sarker IH. Machine learning: Algorithms, real-world applications and research directions. SN Comput Sci 2021;2(3).160. https://doi.org/10.1007/s42979-021-00592-x
13. Zarkowsky DS, Stonko DP. Artificial intelligence’s role in vascular surgery decision-making. Seminars in Vascular Surgery 2021;34(4):260–7. https://doi.org/10.1053/j.semvascsurg.2021.10.005
14. Tobin J. Artificial Intelligence: Development, risks and regulation [Internet]. 2023 [cited 2023 Aug 31]. Available from: https://lordslibrary.parliament.uk/artificial-intelligence-development-risks-and-regulation/
15. Powles J, Hodson H. Google DeepMind and healthcare in an age of algorithms. Health and Technology 2017;7(4):351–67. https://doi.org/10.1007/s12553-017-0179-1
16. Tran Z, Byun J, Lee HY, Boggs H, Tomihama EY, Kiang SC. Bias in artificial intelligence in vascular surgery. Seminars in Vascular Surgery 2023;36:430-4 https://doi.org/10.1053/j.semvascsurg.2023.07.003
17. Lee MH-Y, Li B, Feridooni T, et al. Racial and ethnic differences in presentation severity and postoperative outcomes in vascular surgery. J Vasc Surg 2023;77(4). https://doi.org/10.1016/j.jvs.2022.08.043
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(3):179-185
Publication date:
May 23, 2024











