REVIEW
Safety and efficacy of tranexamic acid in major non-cardiac vascular surgery: a systematic review and meta-analysis
Atha K,1 Corrigan L,1 Bera K,2* Shah A3,4,5*
Plain English Summary
Why we undertook the work: Tranexamic acid (TXA) is a medicine used to reduce bleeding in people undergoing major surgery by stabilising blood clots. However, too much clot can obstruct blood flow to important organs such as the heart, lungs and brain. There is a delicate balance between the formation and breakdown of clots. Using drugs such as TXA can help reduce bleeding and avoid the need for blood transfusion, but there are some concerns that these clots may cause harm by limiting blood flow to important organs. So far, TXA has been shown to reduce bleeding without causing clot-associated problems in patients having open heart surgery, women giving birth and in those who are injured from trauma. However, TXA has not been used very much in vascular surgery (surgery on major blood vessels of the body) because the procedures (which include applying clamps to stop blood flow) are per se more prone to clot formation. The aim of this research was to assess the safety and benefits of TXA in people having vascular surgery.
What we did: We searched seven large research databases for studies that looked at TXA versus a placebo (dummy treatment) or no TXA. We were interested in studies that looked at adult patients. We studied and summed up the results of these studies.
What we found: We found three studies. These included a total of 1,560 adults, which compared TXA to normal saline (salty water) in patients having vascular surgery. In two studies the TXA was given in a vein and in one study it was given as tablets. We found that TXA given to people through a drip does not increase the risk of clot-related problems such as heart attacks or blood clots in the legs. On the other hand, we found no evidence that TXA reduced severe bleeding (eg, major organ bleeds, fatal bleeds) either. However, our confidence in these results is low. One study investigated the use of TXA tablets in patients who had their aorta repaired. The use of TXA for 30 days in these patients had no effect on blood leakage around the repair site. This study also did not find that TXA increased the number of clot-related problems.
What this means: The information from our research suggests that TXA does not increase the risk of clot-related problems in patients having vascular surgery. However, the current studies are limited by small numbers of patients. This means that we do not have enough information to say for certain whether or not we should use TXA in vascular surgery. Ongoing and future studies will help reduce this uncertainty and provide more definitive information for doctors and patients.
Abstract
Background: Tranexamic acid (TXA) is a synthetic lysine analogue that inhibits fibrinolysis. The effectiveness of TXA in obstetrics, trauma and orthopaedic and cardiac surgery is well established. However, concerns regarding its prothrombotic potential remain, which is an important consideration for vascular surgery. We aimed to evaluate the safety and efficacy of TXA in adults undergoing major non-cardiac vascular surgery.
Methods: A pre-specified protocol (PROSPERO CRD42023427282) was followed. Relevant databases (PubMed, MEDLINE, EMBASE) were searched for randomised controlled trials (RCTs). Data extraction and risk of bias assessments were performed in duplicate. A random effects model was used to synthesise data from RCTs. Measures of effect were reported as relative risk (RR) with 95% confidence intervals (CIs). The primary safety outcome was a composite of arterial and venous thromboembolic events (composite of myocardial infarction, non-haemorrhagic stroke, peripheral arterial thrombosis and symptomatic proximal venous thromboembolism). Certainty of evidence was assessed using the GRADE approach.
Results: After screening 1989 records, three RCTs were included, cumulatively enrolling 1,560 participants. In all trials, patients received either TXA (intravenously or orally) compared with placebo. There was no evidence of the effect of intravenous TXA on thromboembolic events (RR 1.10, 95% CI 0.89 to 1.36, two RCTs, 1460 participants, low certainty of evidence) or on critical bleeding (composite of life-threatening, critical and major organ bleed) (RR 0.85, 95% CI 0.65 to 1.11, two RCTs, 1460 participants, low certainty of evidence). TXA may reduce postoperative blood loss, especially at 0–4 hours (Cliff’s delta −0.41, 95% CI −0.19 to −0.59) and 0–24 hours (Cliff’s delta −0.37, 95% CI −0.14 to −0.55) after surgery. There was no evidence of an effect of TXA on reducing perioperative red blood cell (RBC) transfusion requirements (RR 0.66, 95% CI 0.11 to 3.95, one RCT, 100 participants). One RCT assessed oral TXA and found no evidence of an effect on type II endoleak post endovascular aneurysm repair. No thrombotic complications were reported in this RCT during the study period.
Conclusions: We found no evidence of an effect of TXA on thromboembolic complications. While TXA appears to reduce early postoperative bleeding, the clinical relevance of this is uncertain. Due to limitations of study design and the variety of vascular procedures, the role of TXA in vascular surgery is still unclear. Ongoing trials may reduce this uncertainty. Registration: Prospectively registered on PROSPERO (CRD42023427282)
Introduction
Tranexamic acid (TXA) is a synthetic lysine analogue that inhibits the conversion of plasminogen to plasmin, thereby inhibiting fibrinolysis.1 Large pragmatic randomised controlled trials (RCTs) have demonstrated that TXA is associated with a reduction in mortality in patients with major traumatic haemorrhage,2 postpartum haemorrhage3 and mild to moderate traumatic brain injury4; reduced transfusion requirements in adult and paediatric cardiac surgery5,6; and caesarean delivery7; and fewer critical bleeding events in major non-cardiac surgery.8 TXA is on the World Health Organization’s list of essential medicines.9
Despite evidence of efficacy in these settings, owing to the mechanism of action of TXA, concerns about thromboembolic risk remain. This risk may be more pertinent in patients with active thromboembolic disease, inappropriate administration or those with unbalanced haemostatic systems favouring thrombosis. In a large RCT enrolling patients with gastrointestinal bleeding, TXA was associated with an increased risk of venous thrombosis with no effect on mortality.10 Possible reasons for these findings include the high dose of TXA administered (4 g over 24 hours) and the delayed presentation to hospital of many of the patients, missing the early period of excessive fibrinolysis.
Patients undergoing major non-cardiac vascular surgery are per se more prone to thrombosis than general surgery patients.1,11 Hypercoagulability caused by TXA can potentially cause damage to – and occlude arteries recently operated on – new bypass grafts or endovascular stents and stent grafts, which can lead to rare but devastating complications such as limb loss and even death.12 Although systematic reviews have not demonstrated an increased risk of thromboembolic complications associated with TXA in surgical and non-surgical settings,13,14 these often include heterogeneous cohorts of patients. Therefore, we aimed to evaluate the safety and efficacy of perioperative TXA (any dose or route) focusing only on patients undergoing major non-cardiac emergency or elective vascular surgery.
Methods
This systematic review followed a protocol that was designed and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Appendix 1 online at www.jvsgbi.com).15 The protocol is registered on PROSPERO (CRD42023427282). Ethics committee approval was not required as this was a synthesis of previously published literature.
Information sources and search strategy
Relevant bibliographic databases including PubMed, EMBASE (via OVID), MEDLINE and Transfusion Evidence Library, were searched. The full search strategy (see Appendix 2 online at www.jvsgbi.com) included terms relating to or describing exposure (TXA), outcome (thrombosis, bleeding) and the study population (adults undergoing major non-cardiac vascular surgery). There was no restriction on the date of publication and only peer-reviewed RCTs published in academic journals were included.
Inclusion and exclusion criteria
Eligible RCTs included adult participants (as defined by the study authors) undergoing major non-cardiac vascular surgery and receiving either TXA (any route, dose and formulation) or placebo/no TXA during the perioperative period. Major non-cardiac vascular surgery was defined as including the following elective, urgent and emergency procedures: open or endovascular aneurysm repair of the abdominal aorta (AAA repair or EVAR), thoracic aorta, thoraco-abdominal aorta or lower limb artery; open or endovascular repair of aorta (thoracic, abdominal, thoraco-abdominal) dissection; infrainguinal lower limb bypass surgery (including open and hybrid operations); major lower limb amputation (below, above and through knee amputations); carotid endarterectomy. Cohort studies (prospective and retrospective), case-control studies, letters, editorials, commentaries and case reports were excluded.
Outcomes
The primary outcome of interest was the risk of perioperative arterial and venous thromboembolic complications, which we defined as a composite of myocardial infarction, non-haemorrhagic stroke, peripheral arterial thrombosis and symptomatic proximal venous thromboembolism. Secondary outcomes that were analysed are as follows: critical bleeding (composite of life-threatening, critical and major organ bleed), measured perioperative blood loss, risk of receiving allogeneic blood transfusion in the perioperative period and in-hospital mortality. Given the concerns about thromboembolism associated with TXA in vascular surgery, safety was designated as the primary outcome for this review. Efficacy was chosen as the secondary outcome as it is safety concerns, rather than doubts about efficacy, that we hypothesise have deterred the use of TXA in vascular surgery.
Study selection and data collection process
Data from eligible studies were extracted using a pre-piloted spreadsheet. Extracted data included the number of participants in each study, participant characteristics, study duration, type of vascular operation undertaken, route and method of TXA and control administration, transfusion thresholds utilised (if stated), whether any additional co-interventions were used (eg, cell salvage), as well as information relating to quality assessment. This was done in duplicate by two authors (KA and LC) and disagreements were reviewed by a third author (KB or AS). For studies that did not report data on certain outcomes of interest, the relevant authors were contacted.
Risk of bias assessment
The Cochrane Collaboration’s domain-based risk of bias (ROB) tool16 was used to assess risk of bias in each included study. Each of these parameters was evaluated for each study and scored on a 3-point scale corresponding to a low, unclear or high risk of bias. This was also done in parallel by two reviewers (KA and LC) and disagreements were resolved by a third author (KB or AS).
Data synthesis
Data were analysed using Cochrane Collaboration’s Review Manager 5 (REVMAN 5) software. Wherever possible, a random-effect model was used to synthesise data about primary and secondary outcomes. Measures of effect were reported as relative risk (RR) with 95% confidence intervals (CIs). Each outcome was evaluated as relevant deviations from the control group. When comparing end points of interest, statistical significance was established at a threshold of p<0.05. Heterogeneity between studies was assessed with the use of I².17 Any outcome data that could not be combined into a meta-analysis were synthesised narratively instead.
Assessment of the certainty of the evidence
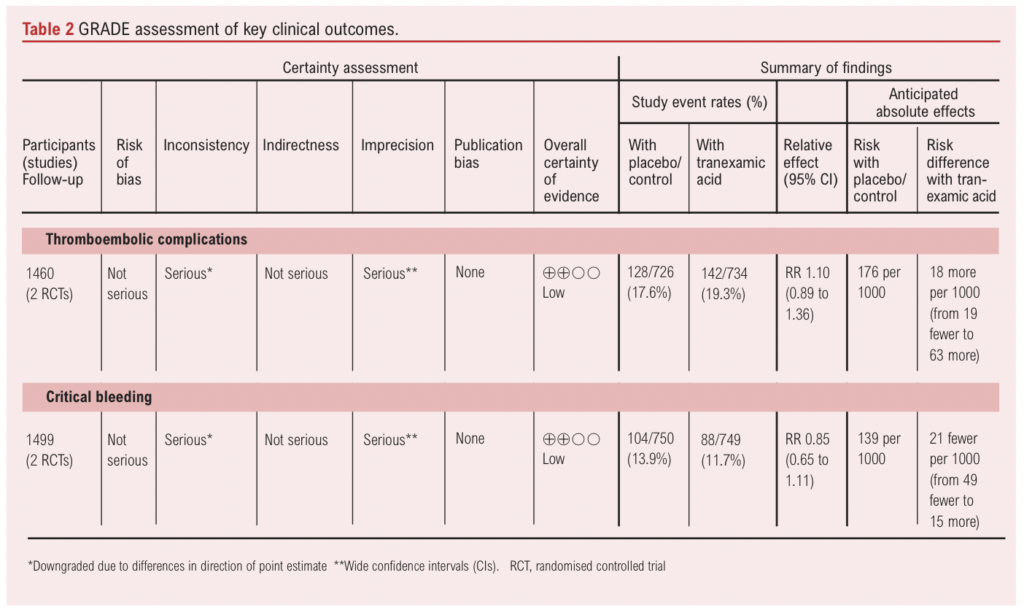
The GRADE (Grades of Recommendation, Assessment, Development, and Evaluation)18 approach was used to assess the overall quality of evidence for the key outcomes of thromboembolic complications and critical bleeding.
Results
Study selection
Our electronic search identified 1,989 relevant publications. Following title and abstract screening, eight articles were shortlisted. Subsequently, full texts were reviewed and three RCTs8,11,19 were ultimately included in this review (Figure 1). The reasons for excluding the five studies were: incorrect study design (ie, retrospective studies) (n=2), full texts in non-English language (n=2) and ongoing clinical trial (n=1). Details of the included RCTs are shown in Table 1. We found one ongoing RCT (NCT04803747) that is planned to complete recruitment in 2024.
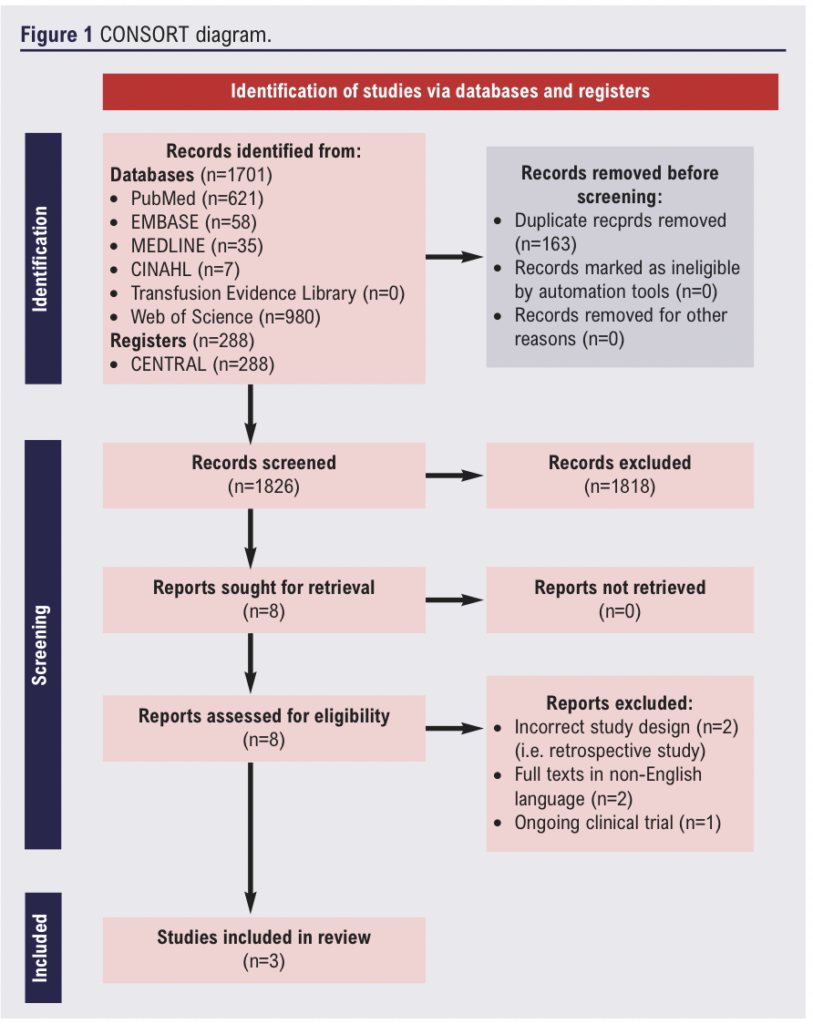
Study characteristics
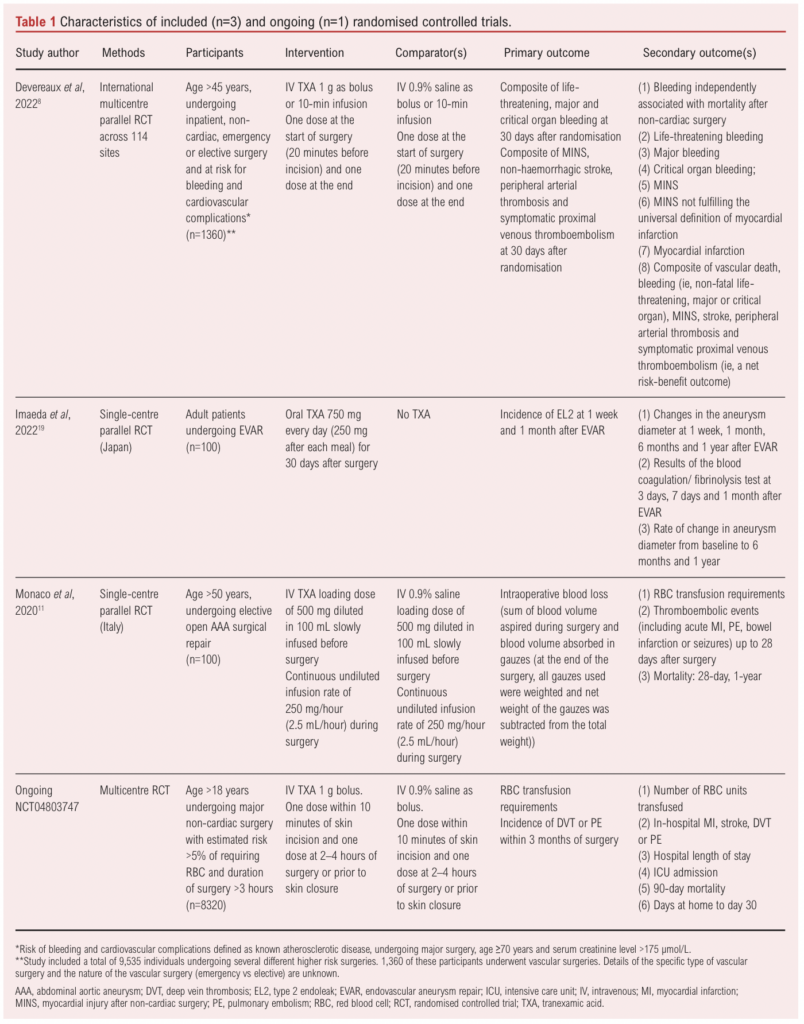
The three included RCTs enrolled a total of 1,560 patients across 24 different countries (Australia, Austria, Belgium, Brazil, Canada, Chile, China, Denmark, France, Germany, Hong Kong, India, Italy, Japan, Malaysia, Netherlands, New Zealand, Pakistan, Poland, Russia, South Africa, Spain, UK and USA). There was one large multicentre international RCT which provided data on 1,360 participants who underwent vascular surgery.8 In comparison, the other two RCTs included 100 participants each.11,19
In two RCTs the patients were administered intravenous TXA at a loading dose of 1 g preoperatively along with a continuous infusion intraoperatively or a bolus at the end. The third trial administered 750 mg oral TXA daily for a month after surgery (Table 1). Two of the RCTs only included patients undergoing elective AAA repair11 and elective EVAR19 and the other RCT included patients undergoing both elective and emergency vascular surgery but data on specific types of surgery were not available.8
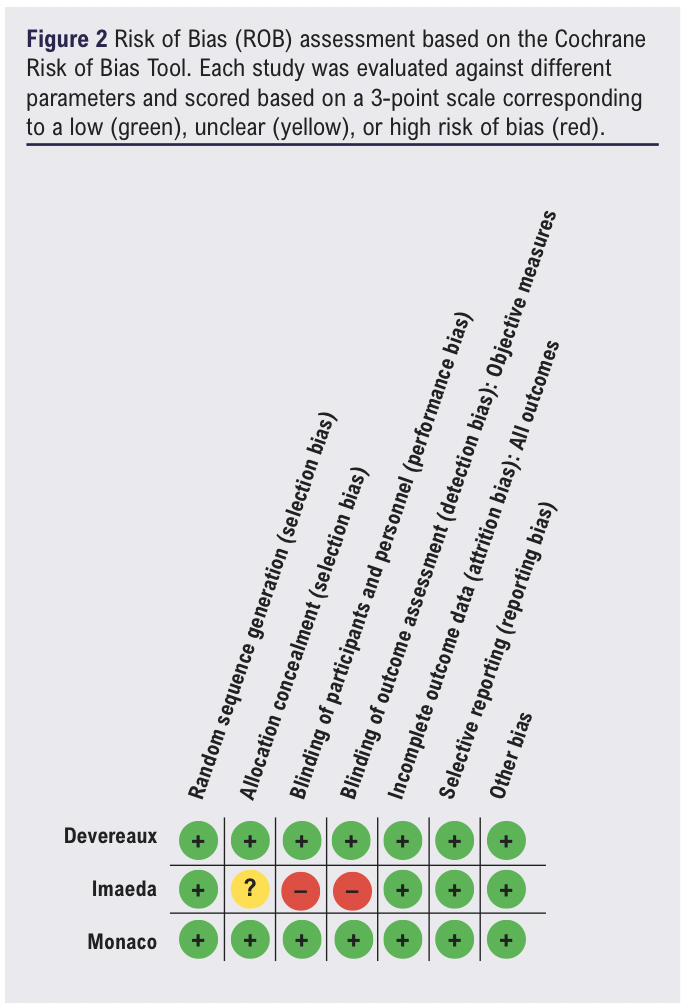
Risk of bias assessment
Two of the RCTs had a low risk of bias across all domains (Figure 2)11 and one RCT was judged to be at high overall risk of bias due to concerns regarding allocation concealment and participant and assessor blinding.19
Primary outcome
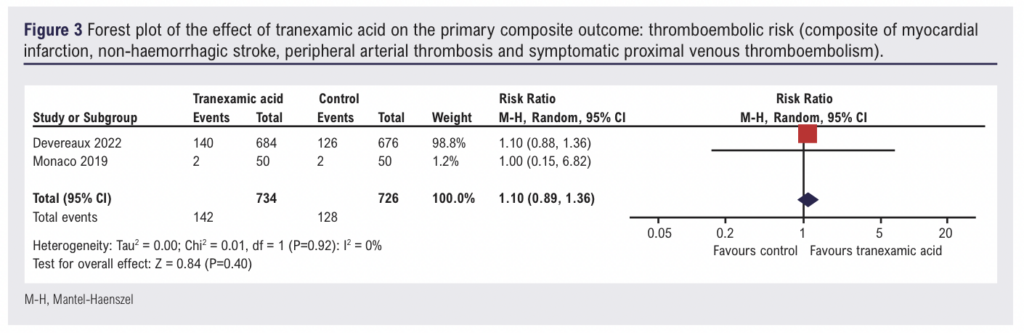
Thromboembolic risk
Two studies (1,460 participants) reported data on the incidence of thromboembolic events in patients receiving intravenous TXA.8,11 Overall, when the data were pooled, there was no evidence of an effect of intravenous TXA on the incidence of thromboembolic events (RR 1.10, 95% CI 0.89 to 1.36) (Figure 3). On the basis of the GRADE framework, this finding was judged to be low certainty evidence (Table 2). One study (85 participants) evaluating oral TXA observed no thrombotic events during the study period.19 Given the variability in the methodology (ie, intravenous vs oral TXA), the study reporting results from oral TXA use was not included in the meta-analysis.
Secondary outcomes
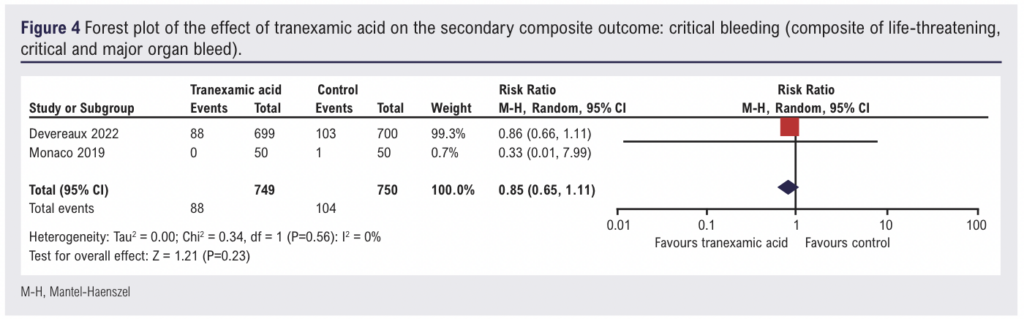
Critical bleeding
Two studies (1,460 participants) reported data on the incidence of critical bleeding in patients receiving intravenous TXA. Overall, there was no evidence of an effect of intravenous TXA on the incidence of critical bleeding (RR 0.85, 95% CI 0.65 to 1.11, low certainty of evidence) (Figure 4).
Blood loss
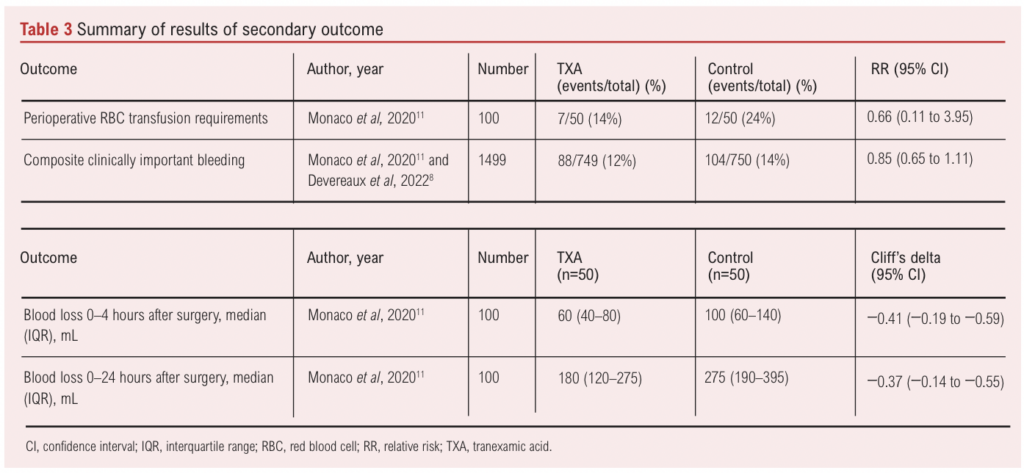
Only one study reported information on blood loss during the first 4 and 24 hours postoperatively.11 During the first 4 hours, participants who received TXA lost a median (IQR) of 60 (40–80) mL blood compared with participants who received placebo who lost a median (IQR) of 100 (60–140) mL blood (Cliff’s delta –0.41 (–0.19 to –0.59), p<0.001). Similarly, during the 24 hours post-surgery, participants receiving TXA continued to have a significantly lower volume of blood loss compared with those who received placebo (Cliff’s delta –0.37 (–0.14 to –0.55), p=0.002) (Table 3).
Risk of receiving allogeneic RBC transfusion
One study (n=100) reported data on perioperative blood transfusion requirements and found weak evidence of an effect of TXA on reducing transfusion requirements (RR 0.66, 95% CI 0.11 to 3.95) (Table 3). Devereaux et al8 reported on allogeneic RBC transfusion across all included surgical specialties (HR 0.77, 95% CI 0.68 to 0.88) but did not provide transfusion data specific to vascular surgery. We contacted them for additional data regarding the vascular subgroup but they were unable to share it at this time.
In-hospital mortality
One study (n=100) provided data on in-hospital mortality and did not report deaths in either study arm.
Oral TXA
Imaeda et al evaluated the use of oral TXA for the prevention of type II endoleak in adults undergoing EVAR (n=100).19 Acknowledging the heterogeneity in the administration of intravenous and oral TXA as well as the variability in reported outcomes, we have narratively summarised the findings of this trial. The study reported that seven days post EVAR, type II endoleak was observed in 14 patients (34.1%) in the TXA group and in seven patients (15.9%) in the non-TXA group. At 1 month follow-up post EVAR, 12 patients (29.3%) in the TXA group and six patients (13.6%) in the non-TXA group were found to have type II endoleaks. There was no significant difference between the two groups in the incidence of type II endoleak (p=0.051 and p=0.08). No adverse events such as thrombus formation due to oral TXA were observed during the study period.
Discussion
The key findings of this systematic review are: (1) there is no evidence of an effect of TXA on thromboembolic events or on critical bleeding in patients undergoing major non-cardiac vascular surgery (low certainty of evidence); (2) while TXA appears to reduce early postoperative bleeding, the clinical relevance of this remains unclear, particularly in an era of patient blood management interventions20 such as cell salvage, restrictive transfusion and minimally invasive surgical techniques; and (3) the current evidence base is limited by the small number of RCTs and wide CIs around the effect estimates, which could encompass clinically important differences.
Both bleeding and thrombosis in patients undergoing vascular surgery are associated with poor outcomes and the underlying pathophysiology and mechanisms are multifactorial. Perioperative risk factors for bleeding include systemic anticoagulation with heparin (to prevent graft thrombosis or clot extension), preoperative use of antiplatelets and/or anticoagulants, intraoperative hypothermia, cross-clamp position, haemodilution and consumption coagulopathy during ongoing blood loss.21 Similarly, a procoagulant state has been observed in AAA repair with increased thrombin generation and inhibition, which may lead to microvascular and macrovascular thrombosis. This can eventually manifest as myocardial infarction, graft thrombosis, multi-organ failure and venous thromboembolism.22 The presence of hypercoagulable comorbidities is associated with an increased risk of developing venous thromboembolism and graft thrombosis in patients undergoing vascular surgery.23,24
The incidence of perioperative RBC transfusion in patients undergoing vascular surgery ranges from 0% to 25%.25,26 Both excessive bleeding and perioperative RBC transfusion are associated with increased postoperative morbidity and mortality.25,27
Certain patient groups such as those with preoperative anaemia, Jehovah’s Witnesses or risk factors for bleeding (eg, anticoagulants) may benefit from TXA, but further research is needed.
Operations at risk of moderate-to-severe blood loss (at least 500 mL) where the use of TXA is recommended by national guidelines include open AAA repair, complex lower limb bypass surgery and major lower limb amputation.28 However, a survey of anaesthetists in Australia and New Zealand found considerable variability in the use of TXA in vascular procedures. While 3% of vascular anaesthetists routinely administered TXA, 40% did not administer it at all and 20% and 37% reported administering TXA selectively and on surgical request, respectively.29 The reasons for this are unclear but may relate to concerns regarding thrombosis. It is worth noting that, in the study by Devereaux et al,8 TXA did not meet the non-inferiority margin for the thromboembolic composite safety outcome, although some have argued that the upper bound of the CI (1.14) just surpassed the non-inferiority margin (1.125) and that it is likely that the true effect lies below this margin.30 Others have expressed concerns regarding widespread prophylactic use in the absence of established fibrinolytic bleeding, and that a more individualised approach may be needed.16,31 It is unclear whether similar patterns exist in the UK or elsewhere and how they may differ between elective and emergency surgery cases.
Our findings are broadly in agreement with recent work by Tsan et al,13 although they did not include data from Devereaux et al8 in their subgroup analysis for vascular surgery. The strength of this review is the strict methodological process. We followed Cochrane Collaboration, PRISMA and GRADE recommendations. We conducted a comprehensive search of multiple databases and clinical trial registries to ensure all relevant studies would be captured. All screening, data extraction and risk of bias assessments were done in duplicate. Limitations of this review stem from the limited number of studies included. There were only three eligible studies and only two were included in the meta-analysis given methodological and reporting differences between trials. Once available, we aim to update this review with data from an ongoing study (NCT04803747) to reduce the uncertainty on the benefits and risks of TXA in patients undergoing major non-cardiac vascular (or other) surgery.
The 2023 NHS Blood and Transfusion (NHSBT) National Comparative Audit in the UK found that only 900 out of 1,336 surgical patients were given TXA. Around one-third (32.6%) of patients who were eligibile for it did not receive it.32 This prompts consideration of factors that are preventing clinicians from using TXA, even when it has been shown to reduce major bleeding by 25% without increasing the risk of thromboembolic events. Additionally, the audit also reported “the low use of TXA in vascular surgery noteworthy (13.5%)”. We hypothesise that this may be explained by the paucity of evidence that we have identified in this systematic review. Evidently, the currently available data on the use of TXA in vascular surgery are insufficient to definitively guide clinical practice either for or against its use.
The publication of the Infected Blood Inquiry report along with recent national blood shortages has again re-ignited efforts to promote the use of TXA. Example recommendations include incorporating TXA in preoperative WHO checklists.33,34 It is likely that a key reason TXA is not widely used in vascular surgery is due to safety concerns rather than issues of oversight during procedures. Hence, until these concerns are addressed, vascular surgeons and anaesthetists are unlikely to start using TXA merely because it is included in preoperative checklists.
At the moment there are no ongoing trials studying TXA solely in patients undergoing vascular surgery. However, if such a trial were to be conducted, it would need to include patients undergoing open procedures at high risk of blood loss. The end points of this trial should assess both safety (incidence of thromboembolic events) and efficacy (effects on major bleeding and RBC transfusion requirements). Future trials should also consider ‘enrichment’ strategies by enrolling patients at high risk of experiencing the outcomes of interest (eg, major bleeding, thrombosis) such as those on antiplatelet and anticoagulant therapy, scheduled to undergo complex surgery and likely to require prolonged aortic cross-clamping duration, and those with multiple comorbidities. Although a pragmatic TXA dose of 1 g is often used, the optimal dose, route and timing is still a topic of active research. For the time being, our data may provide some reassurance to vascular anaesthetists and surgeons with regard to the risk of thromboembolic complications, but further research is needed.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(4):194-202
Publication date:
August 26, 2024
Author Affiliations:
* Contributed equally
1. Medical Student, University of Oxford Medical School, Oxford, UK
2. Senior Clinical Fellow, Department of Vascular Surgery, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
3. Senior Clinical Research Fellow, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK
4. NIHR Blood and Transplant Research Unit in Data Driven Transfusion Practice, Radcliffe, Department of Medicine, University of Oxford, Oxford, UK
5. Consultant Anaesthetist, Department of Anaesthesia, Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, UK
Corresponding author: Akshay Shah Nuffield Department of Clinical Neuroscience, Level 6 West Wing, John Radcliffe Hospital, Oxford OX3 9DU, UK Email: akshay.shah@ ndcn.ox.ac.uk











