PROTOCOL
Surgical Site Infections in Major Lower Limb Amputation: An International Multicentre Audit (SIMBA): Study Protocol
Fabre I on behalf of the SIMBA Study Group*
Plain English Summary
Why we are undertaking this work: In the UK in 2022 over 3,000 people needed an amputation of their leg. This meant they lost their leg above the ankle. This was mainly due to poorly controlled diabetes and blocked arteries that supply the foot and leg. Following amputation surgery, one of the risks is getting an infection of the wound. Wound infection can sometimes be minor, just needing antibiotic tablets to treat. Sometimes it can be serious, needing more surgery or even resulting in death. We do not know how many people get a wound infection after an amputation. We also do not know how infection affects patients, their loved ones or healthcare systems. Many things are used (eg, different dressings and stitches) to try to reduce the occurrence of wound infections, but we do not know if they are actually effective. Two large organisations in the UK have produced guidance designed to reduce infections and improve patient outcomes following amputation. It is also unknown if hospitals are using such guidance.
What we will do: To find out more about the rates of infection and how to reduce it we have designed a large multicentre audit across the whole of the UK and other countries. This will be known as the Surgical Site Infection in Major Lower Limb Amputation (or SIMBA) audit. We will record details of as many patients as possible undergoing amputation surgery. This will happen over 8 months from October 2023 to May 2024. Taking part in the SIMBA project will not affect the care of a patient. We are simply recording what happens to people during and after surgery.
What this means: We hope this will help us find out ways in which we can improve care for those undergoing amputation in the future. The results of the SIMBA audit will be reported to major organisations and charities involved in the care of patients who have amputations.
Abstract
Background: Over 3,000 major lower limb amputations (MLLA) occur in the UK per annum. A significant proportion of patients following MLLA will go on to develop a surgical site infection (SSI). SSIs can range from a simple superficial infection that is treated with oral antibiotic therapy to deeper infections which can lead to wound dehiscence and, ultimately, surgical revision. SSIs can have a significant impact on patient mobility, function, morbidity and mortality as well as wider effects on carers, community services and hospital systems. Despite these potential impacts there are limited data to determine the rate of SSI in patients undergoing MLLA, adjuncts that successfully prevent SSI, factors that predispose patients to SSI and compliance with national guidance set out by key stakeholders in vascular surgical care.
Methods: To address this gap in evidence we propose a large, international, prospective, collaborative audit that aims to compare current practice against recommendations set out by the National Institute of Health and Care Excellence and The Vascular Society of Great Britain and Ireland and to determine the frequency of significant outcomes related to SSI (as defined by the Centre for Disease Control) in consecutive patients undergoing MLLA over an 8-month period including the incidence of SSI, wound dehiscence and surgical revision at 30 days, frequency of use of adjuncts designed to reduce SSI and predictors of SSI. Outcomes will also be captured at 1 year post-MLLA if funding permits.
Discussion: This multicentre audit will allow us to describe the incidence and burden of SSI and wound dehiscence in patients undergoing MLLA. The strengths of this audit will lie in its use of contemporaneous data collection from numerous hospitals and the in-depth data collection focusing primarily on MLLA SSI. It is anticipated that the audit will provide impactful data for future comparisons with global practice and support the design of robust and meaningful studies.
Please see supplementary material (online at www.jvsgbi.com) for a visual abstract.
Introduction
Background and rationale
Surgical site infection (SSI) is a significant potential complication of any surgical procedure, acknowledged by the National Institute of Health and Care Excellence (NICE) as a leading cause of in-hospital morbidity and mortality.1 In vascular surgery, patients undergoing major lower limb amputation (MLLA) may be at an increased risk of developing SSI due to underlying risk factors including ischaemia, pre-existing infection and diabetes.2 The Vascular Society of Great Britain and Ireland (VSGBI) provides a best practice clinical care pathway designed to optimise quality of care to reduce the risk of complications.3 NICE have also published guidance relating to the prevention and treatment of SSI.4
Evidence reporting the incidence of SSI in patients following MLLA due to vascular conditions is currently limited. Single-centre studies have previously reported an SSI rate of up to 27% in vascular patients.5-8 Due to relatively high rates of SSI, many adjuncts have become available to surgeons such as antimicrobial dressings and negative pressure therapy. However, many of these have little evidence of clinical or cost-effectiveness for their use, particularly in MLLA wounds. Evidence for the use of any adjuncts to care other than a prolonged 5-day course of intravenous antibiotics following MLLA is sparse.9,10 Furthermore, there is no consensus on the most effective operative practices – for example, wound closure technique or placement of drains to minimise the occurrence of SSI in MLLAs.
The importance of this issue has been recognised by both patients and multidisciplinary clinicians. The Priority Setting Partnership led by the Vascular Society of Great Britain and Ireland (VSGBI) in conjunction with the James Lind Alliance identified improving clinical outcomes for patients undergoing MLLA and improving wound healing as two of the top research priorities.11
In patients undergoing MLLA, the baseline SSI rate as well as compliance with national guidance is currently unknown. In 2022, VSGBI estimated that 3086 MLLAs were undertaken in UK vascular units. Given SSI rates were estimated at 40%,12 this potentially represents a huge impact on patients, carers and hospital systems. To clarify the baseline SSI rate, adherence to national guidance and assess the adjuncts currently in use for SSI reduction, we have created the ‘Surgical Site Infections in Major Lower Limb Amputation’ (SIMBA) international multicentre audit.
Objectives
• To compare the performance of units with NICE guidance relating to SSI prevention specifically related to MLLA4
• To capture centre-specific data regarding pathways and policies surrounding MLLA and compare this with the VSGBI Best Practice Clinical care pathway for MLLA (for patents under the care of a vascular surgeon)
• To calculate a 30-day incidence of SSI post-MLLA
• To calculate a 30-day incidence of wound dehiscence post-MLLA
• To identify the cause of wound dehiscence post-MLLA (eg, ischaemia or haematoma)
• To calculate a 30-day incidence of revision surgery post-MLLA (to the same or higher level)
• To identify the patient and surgical risk factors associated with MLLA SSI
• To calculate the incidence of early complications related to SSI including sepsis, acute kidney injury, all-cause mortality, increased length of hospital stay or admission to critical care.
• To capture 1-year outcome data for these patients (mortality, amputation revision, ambulation status) and assess the impact of SSI on these outcomes.
Project design
• Multicentre international prospective audit of current practice.
• Non-interventional, only routinely collected data will be collected.
Methods
Participants, interventions and outcomes
Project setting
SIMBA will be disseminated via the Vascular and Endovascular Research Network (VERN). VERN is a trainee-led national research collaborative that is run by, and engages with, research-active vascular trainees and allied healthcare professionals, and has expertise in running national and international audits of practice.
Hospitals providing emergency and/or elective MLLA surgery in the UK and abroad will be recruited via VERN. MLLA surgery can be performed within a vascular surgery department, orthopaedic department or other appropriate department. Based on current interest, more than 30 units are expected to be enrolled, with an expected sample size of around 1000 records. Whilst the best practice policies are based on UK documents, SIMBA will also capture how non-UK centre practice aligns to these guidelines.
Eligibility criteria
SIMBA will capture data on consecutive patients undergoing MLLA. Any patient undergoing MLLA due to complications of peripheral arterial disease (PAD), diabetes mellitus, trauma, cancer and other reasons are eligible for enrolment if they meet the specified inclusion criteria below. Eligible patients will be identified by screening data available to clinical teams; patients will not be approached/contacted and enrolment in SIMBA will not affect care decisions or choices. In patients undergoing MLLA of both limbs during the duration of SIMBA data capture, so long as the patient is eligible both sides will be included (as separate case records).
Inclusion criteria:
1. Patients aged 18 years and above.
2. Patients undergoing primary MLLA (including the following cases: elective/non-elective, hip disarticulation, above knee, through knee/Gritti-Stokes or below knee amputation)
3. Emergency or elective MLLA revision surgery (defined as any revision surgery which requires shortening of the bony length of the residual limb)
Exclusion criteria:
1. MLLA with complex reconstruction (eg, myocutaneous flap) to provide coverage of the amputation site (this does not encompass patients undergoing myodesis and/or myoplasty)
2. MLLA with a concomitant placement of an osseous integration
3. Staged amputation (defined as a MLLA performed in two or more separate planned visits to the operating theatre)
Outcomes
Data from consecutive patients undergoing MLLA meeting the inclusion criteria will be collected prospectively. Data will be captured for each participant until 30 days following surgery.
Outcomes are based on the short-term core outcome set for MLLA, including problems with amputation healing and infection, mortality, requirement for re-admission, re-operation or further specialist treatment for complications.13 The 30-day postoperative morbidity grade will be recorded as per the Clavien–Dindo scale.14 Outcomes will also include compliance with NICE guidelines on SSI prevention.5
The Centres for Disease Control and Prevention (CDC) define that, for MLLA, SSIs are wound associated infections presenting within 30 days of surgery.15 SSIs will be limited to those apparent to the treating vascular clinicians within 30 days of surgery. It is recognised that this audit may not capture milder infections treated with oral antibiotics or simple topical therapies in the community; this will be accounted for and discussed in the analysis and dissemination of SIMBA.
Outcomes that will be captured for individual patients are shown in Appendix 1 (online at www.jvsgbi.com). Preoperative variables will encompass modifiable and non-modifiable risk factors related to the development of SSI postoperatively including age, sex, body mass index, preoperative haemoglobin, albumin, glomerular filtration rate, presence of diabetes, smoking status, comorbidities, preoperative perfusion status of the limb, existence of open wound(s), concurrent infection and history of prior vascular intervention on the ipsilateral limb. Perioperative data will include grade of operating surgeon and anaesthetist, operative time, estimated blood loss, closure technique and drain placement. Postoperative outcomes include length of hospitalisation, postoperative haemoglobin, incidence of postoperative SSI and wound breakdown within 30 days, and subsequent outcomes of patients diagnosed with SSI including development of sepsis, critical care admission, re-admission secondary to SSI within 30 days, additional interventions needed and mortality rates.
Participant timeline
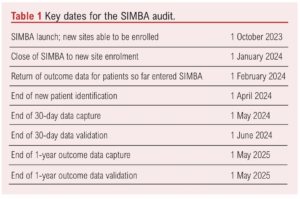
Centre opening will be staggered, and centres will be permitted to start data collection once appropriate approvals are in place. Key dates are shown in Table 1.
Sample size
Sample size will be dependent upon enrolled unit activity and case volume.
Recruitment
SIMBA is required to be registered with each participating centre prospectively, prior to data collection. Within the UK, this is with the audit department. Participating centres outside the UK must comply with local regulations prior to commencement. SIMBA is open to all centres that undertake elective and/or emergency MLLA. In the case of UK vascular units, often they comprise a Hub and Spoke type model.
Each centre will require the support of a named supervising consultant/attending (or equivalent) who will act as guarantor of all activity undertaken at that centre, and a data collection team. The supporting consultant is expected to facilitate the data collection team to secure audit registration (or local research ethics committee/institutional review board approval if required for non-UK countries), provide unit support for engagement, act as guarantor for data capture, validation and upload, provide workplace-based assessment documentation for team members and facilitate local presentation of results.
The local audit team will be responsible for data collection and data validation. This team will comprise a maximum of a supervising consultant/attending and a further six individuals and can include medical trainees, medical students or allied healthcare professionals.
At enrolment each centre will be asked to complete a baseline unit survey. This will collect data on clinical care pathways and policies surrounding MLLA.
Local Information Technology (IT) systems, theatre lists and inpatient ward-based lists will be used to screen for eligible patients at each individual SIMBA centre once registered.
Data collection, management and analysis
Data collection methods
Key demographic data, baseline variables and intraoperative data should be collected as early as possible following MLLA surgery, ideally at the completion of the operation to reduce missing data points.
Postoperative sequelae data points will be collected up until 30 days following surgery. In the case of SSI development, further details will be required regarding extent of infection and subsequent patient outcomes. Such data can be obtained using a variety of routinely collected sources such as: patient hospital notes and electronic records; preoperative assessments, outpatient letters, theatre IT systems, discharge summaries, emergency department and primary care records (where available). Any patient enrolled in SIMBA will not receive additional unplanned follow-up other than what is considered as ‘routine care’ at each site.
Project organisation
The SIMBA Audit is partially funded by the ROSSINI platform as part of the accelerator award scheme (Award ID: NIHR156728).16
The study is coordinated by the Birmingham Centre for Observational and Prospective Studies (BiCOPS) at the University of Birmingham. BiCOPS was established in 2017 and provides methodological support and the infrastructure for the delivery of non-randomised prospective research. BiCOPS has established expertise in the design, coordination and analysis of large-scale national and international cohort studies across a range of clinical specialties. All BiCOPS projects are conducted in compliance with the UK Policy Framework for Health and Social Care Research, Data Protection Act 201817 and the Principles of Good Clinical Practice (GCP). BiCOPS enables the successful delivery of adopted projects through active interaction with national and international networks and collaborative groups.
The SIMBA Study Management Group (SMG) comprises those individuals who have created this protocol and who will be responsible for the day-to-day running and management of the study. This will include the Project leads, SIMBA operations staff, statistician and lead clinicians. The group will meet via regular teleconference to review ongoing progress. The role of the SMG is to monitor all aspects of the conduct and progress of the study, ensure that the protocol is adhered to and take appropriate action to safeguard the quality of the study itself.
In addition to the SMG meetings, the project leads and the BiCOPS staff located within the University of Birmingham will convene monthly for ongoing and continual review of study and progress.
Data management
Source data will be captured and uploaded electronically using an internationally recognised secure web application for building and managing online databases (Research Electronic Data Capture – REDCap).18 It is encouraged that data will be uploaded directly to REDCap as close to the time of surgery as possible. Paper case report forms (CRFs) will be provided to centres to facilitate data capture when direct upload to REDCap is not possible at the time of surgery. No patient identifiable data will be transferred to REDCap. Each local centre will hold a secure database with a minimum of three patient identifiers and a three-digit pseudo-anonymised number used to link perioperative and postoperative data. A template document will be sent to centres on enrolment to be overseen by the local lead who will be responsible for ensuring this file is only stored on-site, is done so securely, and is disposed of appropriately following upload of all follow-up data to REDCap.
Data validation
Data completeness will be quantified following the initial data collection. Any datapoints left blank will be considered incomplete. Data points recorded as “unknown” will count as complete data. Cases with <95% data completeness will be returned to the local centre for completion. If this is not possible, these cases will be excluded from the analysis, as is standard within international collaborative audits.19 Individual patient records with less than 95% completeness of mandatory datapoints will be returned for completion; if this is not possible the patient will be excluded from the analysis. All centres will be required to validate data accuracy in 20% of their uploaded cases (randomly selected); 25% of datapoints (randomly selected) per case will be validated, equating to 5% of total datapoints captured. Any centre reporting accuracy of less than 95% will be required to validate a further 20% of their cases and the lead team member will be asked to investigate and report back to the SIMBA Management Group. Data validation will be undertaken independently by a team member not involved in the initial data collection.
Statistical methods
Simple descriptive analyses will be used to describe variations in practice at registered SIMBA sites. Any continuous data will be tested for normality followed by parametric or non-parametric tests as appropriate. A χ2 test will be used to analyse for differences for categorical variables. Missing data will be analysed to determine the pattern of missingness and, if appropriate, multiple imputation will be carried out using the Markov chain Monte Carlo method. Univariate and multivariate regression analyses will be used to identify independent predictors of 30-day SSI, 30-day wound dehiscence, 30-day stump revision, 30-day all-cause mortality, 1-year all-cause mortality, 1-year stump revision and 1-year ambulation status. Any variable reaching the threshold of p<0.10 on univariate analysis will be entered into a multivariable regression analysis. A p value <0.05 will be considered as statistically significant.
Monitoring
Data monitoring
Data validation comprises confirmation of case ascertainment and data accuracy. At the close of the data capture timeframe, centres will be asked to review theatre logs to ensure that all patients undergoing MLLA during the data collection timeframe were entered. Any patients not included can then be added retrospectively. It is appreciated that not all data may be available retrospectively, but the SIMBA team will account for this during analysis and dissemination.
As SIMBA is an international prospective audit, a data monitoring committee is not formally required.
Harms
As SIMBA is not an interventional study and is concerned only with events related to routine clinical practice, reporting of serious adverse events or similar is not required.
1-Year outcome data
Intention for longer term objectives and outcomes is funding-dependent. Funding will be sought to keep the REDCap database open and permit the follow-up of patients 1 year after their MLLA. This will be to assess the impact of SSIs on longer-term outcomes after MLLA. Data on mortality, ambulation status and need for revision surgery will be collected. If this is feasible, one more team member can be added to the existing team to support the return of 1-year data. It is expected that the overseeing consultant/attending will not change.
Ethics and dissemination
Research ethics approval
The SIMBA methods do not meet the criteria of research as classified by the Health Research Authority decision tool20 (see Appendix 2 (online at www.jvsgbi.com). Therefore, research ethics approval was not sought. Every participating centre will register the audit locally prior to data collection (audit and service provision registration at all NHS sites involved). Centres outside the UK should comply with local regulations.
Protocol amendments
Any amendments to the project or this protocol will be communicated immediately to each site directly. This version and any future versions of this protocol will be uploaded to the VERN website which is readily available to all sites.21
Consent or assent
As SIMBA is a multicentre international audit of practice centred around routine care, individual patient consent is not required. All data entry into REDCap will be completely anonymised as stated in the data management section.
Confidentiality
Only anonymised routinely collected data will be collected. All participant information, data and outcomes will be strictly confidential and only the central research team will have access to the complete dataset. All data will be handled in accordance with the principles of the Data Protection Act 2018 and GDPR.17
Declaration of interests
The SIMBA group have no conflicts of interest to declare.
Access to data
The final SIMBA dataset will be available to members of the core project management group listed in this protocol. Those outside of the study group may access the dataset on reasonable request. The data will be available following publication of the initial SIMBA findings. Data will be stored and accessed in accordance with the principles of GCP.
Dissemination policy
All publications and presentations relating to SIMBA will be authorised by the SMG. The results of SIMBA will be submitted for presentation at national and international meetings. Any manuscript(s) from the resultant data will be submitted for peer-reviewed publication. A writing team, including those involved with design, implementation and dissemination of the audit, and those contributing to data analysis will be responsible for both presentation(s) and publications(s). For both, a collaborative authorship model will be used, with a list of contributors clearly listed at the end of the manuscript. To qualify for citable collaborative co-authorship, individuals must have either:
• Had a significant role in the set-up and management of the audit, including audit department registration, creation of a data collection team and engagement with the SIMBA team to ensure timely upload of data (with validation) and completion of the questionnaire
OR
• Captured sufficient data to warrant authorship – this would be the equivalent of collecting baseline and follow-up data on approximately 10 patients, although it is appreciated individuals may participate in only baseline data collection or only follow-up data capture. Data collection is expected to be complete (>95% variables completed) and submitted in a timely manner
OR
• (For consultants/attendings/senior supervisors) provided oversight and support as detailed in the ‘Recruitment’ section.
OR
• Captured 1-year outcome data sufficient to warrant authorship.
The local lead at each centre will be responsible for ensuring that the SIMBA SMG have the names and contact details of all collaborators who qualify for collaborative co-authorship at their centre. All collaborators will be given the opportunity to review draft manuscript(s) prior to submission. Whilst the SIMBA team appreciates the importance of this step, the team are also keen to ensure this stage does not add to significant delays in submission and dissemination. All collaborators should inform the team of any changes in contact details. Unless there are major issues or questions identified, collaborators will be given a single opportunity to comment on the paper before it is returned to the Writing Group for further review within 72 hours. The Writing Group will make a final decision regarding the comments and edits made during this process.
Plain language summaries will be created and distributed to national amputation charities and key stakeholders.
Discussion
This multicentre audit will allow us to reliably determine the incidence and burden of SSI and wound dehiscence in patients undergoing MLLA. The strengths of this audit will lie in its use of contemporaneous data collection from numerous hospitals, the in-depth data collection focusing primarily on MLLA SSI, and the capture of 1-year outcome data.
It is anticipated that the audit will provide impactful data for future comparisons with global practice and support the design of robust and meaningful studies.
Limitations of the audit will include its inability to define specific causative associations between factors and the incidence of SSI. Therefore, focus will be placed on factors either known to contribute to SSI or areas with limited evidence. Although the VERN Collaborative has experience of data collection from previous studies, it will be impossible to confirm reliable consecutive patient recruitment. Finally, the data will be limited to SSIs that are severe enough to prompt review or referral to secondary care, as per previous international SSI audits.22

*Management Committee and Protocol Writing Group (in alphabetical order):
Miss Nina Al-Saadi, Vascular Trainee, Black Country Vascular Network; Email: [email protected]; Mr David Bosanquet, Consultant Vascular Surgeon and Project Co-Lead, South East Wales Vascular Network, Email: [email protected]; Professor Ian Chetter, Professor of Vascular Surgery, Hull York Medical School, Email: [email protected]; Miss Ismay Fabre, Core Surgical Trainee and Project Co-Lead, South East Wales Vascular Network, Email: [email protected]; Mr Brenig Gwilym, Vascular Trainee, Aneurin Bevan Health Board, Email: [email protected]; Miss Louise Hitchman, NIHR Doctoral Research Fellow, Hull University Teaching Hospitals, Email: [email protected]; Mr Terry Hughes, Research Administrator, Birmingham Centre for Observational and Prospective Studies, University of Birmingham, Email: [email protected]; Mrs Judith Long, Vascular Research Manager, Hull University Teaching Hospitals, Email: [email protected]; Dr Laura Magill, Associate Professor of Clinical Trials, Birmingham Clinical Trials Unit, Email: [email protected]; Professor Thomas Pinkney, Professor of Surgical Trials, Institute of Applied Health Research, University of Birmingham, Email: [email protected]; Mr Matt Popplewell, Assistant Professor of Vascular Surgery, Institute of Applied Health Research, University of Birmingham, Email: [email protected]; Mr Michael Wall, Consultant Vascular Surgeon, Black Country Vascular Network, Email: [email protected].
The SIMBA Study protocol was compiled using the Standard Protocol Items: Recommendations for Interventional Trials (2013) Checklist as a guide where relevant to the nature of the project. Available at: https://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/
If you would like to know more about SIMBA, please contact us by email at: [email protected]
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(2):98-104
Publication date:
February 29, 2024
Author Affiliations:
South East Wales Vascular
Network, University Hospital
Wales, Heath Park Campus,
Cardiff CF14 4YS, UK
Corresponding author:
Ismay Fabre
South East Wales Vascular Network, University Hospital Wales, Heath Park Campus, Cardiff CF14 4YS, UK
Email: Ismay.Fabre3@ wales.nhs.uk











