PROTOCOL
The FraiLTI (Frailty in chronic Limb-Threatening Ischaemia) Protocol
Hickson B,1 Sivaharan A,2 El-Sayed T,2 Baljer B,2 Sillito S,2 Shelmerdine L,2 Nesbitt C,2 James E,3 O’Doherty AF,3 Witham M,4 Nandhra S2
Plain English Summary
Why we are undertaking this work: Frailty is a medical term that describes physical weakness, vulnerability or fragility and is often associated with old age. We are undertaking this study to gain an understanding of the occurrence rates and effects of frailty, progressive loss of muscle mass and strength (sarcopenia) and having multiple health issues (multimorbidity) in patients suffering from a severe form of poor leg circulation called chronic limb-threatening ischaemia (CLTI). This is a serious condition affecting blood flow in the limbs and is usually associated with high rates of losing a limb or even death.
What we will do: In this study (FraiLTI), we recruit patients with CLTI who are undergoing an intervention to improve their poor leg circulation. We assess how common frailty, sarcopenia and multimorbidity are among CLTI patients and explore their influence on clinical outcomes. We are aiming to obtain a comprehensive understanding of the situation at a national scale by gathering data from hospitals throughout the UK.
What this means: The results from the FraiLTI study could provide crucial information on the prevalence of health issues such as frailty, muscle loss and weakness, and multiple health conditions among individuals in the UK with severe leg circulation problems, as well as their impact on overall health. This could enable healthcare professionals to identify high-risk patients who need extra care and attention to improve their outcomes. The study will also offer valuable insights for future research and contribute to the overall improvement of care and
Abstract
Background: Frailty, sarcopenia and multimorbidity are conditions commonly associated with the ageing process, and they are frequently observed in patients with chronic limb-threatening ischaemia (CLTI). Nevertheless, the extent to which these conditions are prevalent within the CLTI patient population has not been adequately examined in the UK. This proposed multicentre observational study aims to investigate the prevalence of these conditions in patients with CLTI and to assess their potential impact on important clinical outcomes including mortality, amputation and quality of life.
Methods: FraiLTI (Frailty in chronic Limb-Threatening Ischaemia) is a multicentre prospective observational study in the UK that that aims to investigate the prevalence of frailty, sarcopenia and multimorbidity associated with CLTI. The secondary objective is to investigate potential correlations between frailty, sarcopenia and multimorbidity, with clinical outcomes such as amputation, mortality, major adverse cardiovascular events and readmission rates within a 90-day period. FraiLTI is led by Newcastle University, supported by the Vascular and Endovascular Research Network (VERN) and funded by the National Institute for Health Research (NIHR) and the Newcastle Hospital Charities. REDCap will be used to collect anonymised patient data. All hospitals with a dedicated vascular centre are eligible to participate. Full ethical approval (21/PR/0750) was granted on 13 July 2021. The study is registered on the International Standard Randomised Controlled Trial Number (ISRCTN) registry.
Anticipated impact of the study: This study has the potential to address critical questions identified by the James–Lind Alliance (JLA) Priority Setting Partnership (PSP) in peripheral arterial disease. It is expected to make a substantial contribution to the creation of a prospective CLTI database, integrating essential data on frailty, sarcopenia and multimorbidity that are not currently captured by other registries, despite their profound impact on patient outcomes. This research could provide pivotal insights into the prevalence of frailty, sarcopenia and multimorbidity among the UK’s CLTI population and their corresponding effects on clinical outcomes. Findings from the study will be shared at global scientific conferences and submitted to be published in peer-reviewed journals.
Introduction
Frailty, a concept gaining significant attention in recent years, is defined as a clinically recognisable state of increased vulnerability resulting from ageing-associated decline in reserve and function across multiple physiologic systems such that the ability to cope with everyday or acute stressors is compromised.1 Frailty leaves patients vulnerable to stressors such as illness, trauma or surgery. The high prevalence of frailty, affecting 43.7% of adults aged 65 and older,2 and its potential impact on health outcomes underscores the importance of this issue.3
The elderly population is particularly affected as they are more susceptible to both frailty4 and cardiovascular pathologies.5 Frailty has been linked as a predictor for inferior postoperative outcomes6 as well as numerous adverse health outcomes including falls, disability, hospitalisation and mortality.7,8 Additionally, frailty is associated with an increased risk of disability, which manifests as limitations in performing activities of daily living (ADL) and impacts a patient’s quality of life (QoL).9,10
Sarcopenia, a key component of frailty, is characterised by skeletal muscle dysfunction that develops gradually and predominantly affects older patients,11 leading to reduced strength and muscle mass.12 Sarcopenia is an independent predictor of mortality following both open and endovascular procedures.13,14
Multimorbidity is another significant factor influencing outcomes in patients with chronic limb-threatening ischaemia (CLTI). Previous research in vascular surgery focused primarily on cardiometabolic comorbidities, highlighting the need to investigate the impact of other conditions beyond the central cardiovascular system. Our preliminary retrospective work has shown that sarcopenia14 and anaemia15 are negatively associated with survival and limb loss following revascularisation surgery for CLTI.
There are some retrospective data in the literature which suggest that frailty affects survival in those undergoing intervention for CLTI in Japan16 and the UK.17 A prospective cohort study from Canada found that frailty was associated with mortality and worsening disability post-intervention. There are also systematic reviews that suggest worse outcomes in a wide range of lower limb vascular operations.18-20 However, thus far, data on this topic in the UK remain single-centre and retrospective.
FraiLTI (Frailty in chronic Limb-Threatening Ischaemia) is a multicentre prospective observational study in the UK which aims to investigate the prevalence of frailty, sarcopenia and multimorbidity and their effect on outcomes following a diagnosis of CLTI. This patient-led study addresses several questions raised by the James–Lind Alliance (JLA) Priority Setting Partnership (PSP) in Peripheral Arterial Disease (PAD), with a focus on exploring potential causes for poor outcomes.21
Methods
Study design
This is a multicentre prospective observational study conducted in UK Vascular Centres led by Newcastle University and supported by the Vascular and Endovascular Research Network (VERN). It is funded by the National Institute of Health Research (NIHR) and the Newcastle Hospitals Charity. Figure 1 is the flow diagram of the study protocol.
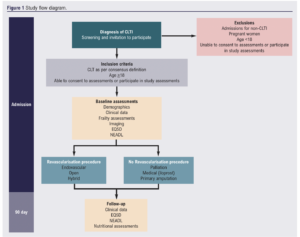
Study population
Inclusion
• All adults aged over 18, able to consent and participate with ongoing assessments.
• All patients with CLTI as per the consensus definition (the presence of PAD in combination with rest pain, gangrene or a lower limb ulceration >2 weeks duration),22 irrespective of pathology, mode of presentation, plan to revascularise, or previous presentations with lower limb arterial disease.
Exclusion
• Admissions for non-CLTI.
• Unable to consent to assessments or participate in study assessments.
• Pregnant women.
• Age <18 years.
Study outcomes
Primary
Prevalence of the following conditions among CLTI patients:
1. Frailty
• Fried’s Frailty Phenotype (FP)
• Rockwood’s Clinical Frailty Scale (CFS)
2. Sarcopenia
• Grip strength
• Skeletal muscle area (SMA) at L3
• Skeletal muscle index (SMI) at L3
(Cut-offs highlighted below)
3. Multimorbidity (>2 long-term health conditions)
Secondary
1. Associations between frailty, sarcopenia and multimorbidity with clinical outcomes, including:
• Mortality
• Major adverse limb events (MALE)
• Major adverse cardiovascular events (MACE)
• Readmission
• Reinterventions
• Discharge destination.
2. Impact of CLTI on patients’ quality of life as measured by validated QoL assessment tools.
These outcomes will facilitate risk prediction, modelling and the identification of potential targets for intervention in future prospective research.
Recruitment
The FraiLTI study is open to all dedicated vascular centres with one team member acting as site lead clinician, who is the point of contact between the FraiLTI study team and the local team. The study is eligible for the NIHR Associate PI Scheme. All patients admitted with CLTI have a diagnosis confirmed by the admitting vascular surgeon. Potentially eligible participants are sign-posted to the relevant research team member only after the patient has suggested they would be agreeable for their details to be shared with a member of the research team on the delegation log at each site. Patients are formally screened according to the inclusion and exclusion criteria. All presentation modes are eligible including outpatient clinic, vascular “hot” clinic, and emergency departments.
The FraiLTI study team provides written and verbal versions of the participant information and informed consent. The local study lead is responsible for ensuring that this process is carried out in accordance with Good Clinical Practice (GCP).
Data collection
Anonymised data collected consists of patient demographics (including postcode for social-economic data), presenting symptoms, previous interventions, and admissions in the past six months.
The research team are collecting patient data in a prospective manner through hospital records, both electronic and paper- based. They then enter the collected data onto a custom-made electronic database hosted on the Newcastle Joint Research Office’s Research Electronic Data Capture (REDCap) platform.
Definitions
Comorbidities are defined as per the American College of Cardiology guidelines23 where possible. Diabetes is defined by documented medical history, and hypertension is defined by documented medical history and use of antihypertensive drugs for this purpose, or systolic blood pressure of at least 140 mmHg or diastolic blood pressure of at least 90 mmHg at admission determined by the average of the first two measurements. Other comorbidities recorded include ischaemic heart disease, stroke, chronic heart failure, atrial fibrillation, hypothyroidism, dementia, anxiety, depression, asthma, chronic obstructive pulmonary disease, osteoarthritis and inflammatory arthropathies.
The following diseases are recorded based on the patient’s documented medical history: ischaemic heart disease or prior myocardial infarction, atrial fibrillation, hypertension, cerebrovascular disease (ischaemic stroke, haemorrhagic stroke or transient ischaemic attack), end-stage renal failure requiring dialysis and chronic obstructive pulmonary disease. Preoperative drugs are also recorded from the pre-assessment clinic documentation. Severity of presentation is measured using both the Rutherford classification and the WIfI score.
Laboratory results
Routine clinical care laboratory test results (including haemoglobin, white cell count, albumin, creatinine and estimated glomerular filtration rate, C-reactive protein and HbA1c) are collected, as well as height and weight.
Sarcopenia assessments
CT angiogram
Axial imaging by means of a CT angiogram (where performed) will enable measurement of the skeletal muscle area (SMA) at the 3rd lumbar vertebra (L3) level (for comparison with previous studies), the mid-thigh and mid-calf levels (to enable detection of regional differences in muscle mass) (see Appendix 1 online at www.jvsgbi.com for details of measurement methodology).
We also calculate a value for the L3 skeletal muscle index (SMI) with the following formula:
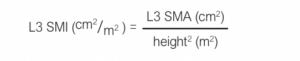
Cut-offs diagnostic of sarcopenia are 134.0 cm2 for men and 89.2 cm2 for women for L3 SMA and 41.6 cm2/m2 for men and 32.0 cm2/m2 for L3 SMI for women.24
Grip strength
Hand grip strength is measured using handgrip dynamometry (see Appendix 2 online at www.jvsgbi.com for detailed methodology).
Frailty assessments
Fried Frailty Index Phenotype
The Fried Frailty Score1 is measured by combining five domains:
1. Weight loss (>4.5 kg in last year).
2. Low grip (<27 kg for men, <16 kg for women).
3. Low walk speed (we anticipate that most participants will have restricted mobility and so will score a point automatically; a cut-off of <0.8 m/s will be used for those who can undertake a 4 m walk test).
4. Exhaustion (measured using two questions from the Centre of Epidemiologic Studies Depression scale (CES-D scale)25 used in the original Fried Score).
5. Low physical activity measured using four activity questions used in the English Longitudinal Study of Ageing.26
A score of >3 or more denotes frailty, 1 or 2 denotes pre-frailty, and zero denotes non-frail.
Rockwood frailty scoring
The Rockwood deficit accumulation model considers frailty as the accumulation of deficits in various domains of functioning including physical, cognitive and social domains.27,28 This study uses the Clinical Frailty Scale, which categorises patients based on their function, morbidity and central nervous system impairment using a clinician’s judgement.28 Studies have verified that the Clinical Frailty Scale is a reliable predictor of negative outcomes.29
Nutrition assessment
We also collect information on activities of daily living, nutritional intake, place of living and mobility aids to provide a comparison for robustness in this disease group.
Quality of life assessment
Participants are invited to complete the Euro-QoL EQ-5D-5L health status assessment30 and Nottingham Extended Activity of Daily Living scale (NEADL)31 (see Appendix 3 (EQ-5D-5L) and Appendix 4 (NEADL) online at www.jvsgbi.com – for examples of the questionnaires).
Follow-up (90 days)
Following the baseline data capture, all patients are followed up irrespective of whether they undergo any revascularisation, and their 90-day outcome data are collected (including mortality, MALE, MACE, respiratory and wound-related complications, readmissions, reinterventions and discharge destination).
Participants are invited to receive a telephone call or postal EQ-5D-5L assessment as well as the NEADL. Food diaries are collected over 2 days (ideally one weekday and one weekend day) (see Appendix 5 online at www.jvsgbi.com).
Data management
Data are collected and uploaded onto a secure REDCAP database platform.
In line with General Data Protection Regulations,32 no identifiable data are uploaded and each patient is assigned a specific audit identification number. The local hospital ID and corresponding audit ID is maintained by the lead clinician at each centre to ensure accurate follow-up data and is securely stored on an appropriate hospital computer.
Data will be kept for two years and then destroyed but will be available to others. A minimum dataset including fully anonymised patient data will be included in the FraiLTI results paper as a supporting information file.
Data analysis
Statistical analysis will be performed using R (R Foundation for Statistical Computing, Vienna, Austria). Normally distributed data will be presented as mean (SD) and hypothesis testing will be performed with unpaired t-tests/Mann–Whitney U tests as appropriate. Categorical data will be analysed by a χ2 test. A p value <0.05 will be considered statistically significant for single comparisons.
Kaplan–Meier survival curves will be used with a log-rank test to compare the overall mortality. Cox proportional hazards regression will be performed; hazards ratios (HR) with 95% confidence intervals (CIs) will be reported along with p values.
Binary logistic regression analysis will be used to identify associations with complications and multiple variates will be tested. The resultant significant variables will be presented as odds ratios (OR) with 95% CIs. An OR of >1 indicates an increased likelihood of the event occurring.
The NEADL scores will be analysed as continuous variables using the statistical tests outlined above. EQ-5D data will be analysed using the “eq5d” R package to provide both descriptive data and longitudinal data to assess how quality of life changes over the course of the study. Techniques to be used include the Paretian Classification of Health Change (PCHC)33 and the Probability of Superiority.34
Nutritional data will be entered into Nutritics Professional Plus v5.81, 2022 software and analysed. The average for each participant will be grouped and then analysed. Total energy intake will be reported as kilocalories (kcal) per day and as a percentage of estimated resting energy expenditure using the Mifflin–St Jeor equation.35 Average total daily intake of macronutrients and micronutrients will be reported. Macronutrients will also be reported relative to body mass. A threshold of 1.2 g/kg body mass/day will be used to identify people with low protein intake.
Regulatory approval and research governance
Full ethical approval (21/PR/0750) was granted on 13 July 2021 for this multicentre prospective observational study. The study is registered on the ISRCTN.
Authorship
This is a national trainee supported research collaborative. It is anticipated that VERN will support the FraiLTI project through one of the streams of collaboration once the regional study is underway. Contributions will be recognised in co-authorship of publications as part of a collaborative research authorship model. This will allow participating clinicians in training to meet the objectives of their training needs whilst providing vital research data, as well as recognising the research activity for the recruiting centre and lead.
Current status
The FraiLTI study recruitment of new centres was between September 2021 and September 2022. The expected date of the last patient to be included is July 2022 and data collection will end in September 2022. The results of this study are expected to be released in early 2024.
Discussion
An internationally agreed definition of frailty remains elusive due to its multifaceted aetiology36,37 and the challenge of distinguishing it from other geriatric conditions.38,39 While frailty is considered a geriatric condition and is closely related to ageing, disability and comorbidity, it is unmistakably different.40,41 For example, despite its higher prevalence among older individuals, frailty cannot be solely attributed to chronological age.36
Research has revealed that frailty is not a static condition but rather a dynamic process that results from the compounded effects of multiple factors.40,42,43 This improved understanding of frailty offers an opportunity to optimise management of underlying factors such as nutritional deficiencies, physical inactivity or chronic diseases, leading to better health outcomes and an improved quality of life.27,42 Ultimately, this is achieved through correct identification and understanding of the scale of the problem through studies such as this one.44
The FraiLTI study represents a pivotal first step in establishing the current prevalence of frailty, sarcopenia and multimorbidity among patients with CLTI in the UK. This initial step is vital to evaluate the scale of the issue and any clinical consequences or associations related to CLTI on a national level. The findings of the study will ultimately inform the development of future intervention studies to improve health outcomes for patients with CLTI, thereby reducing the overall burden of these conditions on the healthcare system.
It is postulated that CLTI patients who also have frailty and/or multimorbidity could be associated with worse clinical outcomes, potentially independent of age. By identifying adverse health outcomes such as quality of life, activities of daily living and mortality, the study aims to enhance the detection of the most vulnerable individuals, thereby improving targeted treatment strategies.
The FraiLTI study is part of a national collaborative research effort led by VERN trainees. By leveraging this platform, the study employs a multicentre design that enables patient recruitment from various locations. This approach boosts the study’s statistical power and increases the potential for a larger sample size. The utilisation of data from diverse sites improves the representation of the UK as a whole, which is crucial when analysing prevalence nationwide. These efforts contribute to more robust and impactful findings that advance the knowledge of CLTI and its correlation with frailty. VERN has a proven track record of producing multinational studies that have a large impact, increasing the credibility of this study.45,46 One limitation of this study will be the inability to determine direct causality between practice and outcome; however, it benefits from being prospective, multicentre and unique in its use of the Fried Frailty phenotype model in this context. Outputs from the FraiLTI study will inform future quality improvement and research projects to improve the care of patients with CLTI.
Pathway to impact
This study is a collaborative effort between patients and clinicians and is supported by the Vascular Society Peripheral Arterial Disease Special Interest Group (PAD SIG). The results will be presented to improve patient care at a national and international level. A writing team, including individuals involved in the design, implementation and dissemination of the FraiLTI study, will be responsible for submitting manuscript(s) for publication(s).
The study will prioritise patient and public involvement, working with the JLA PSP to produce a patient-facing lay summary of the results The JLA and Circulation Foundation will also be notified to promote the summary. Additionally, results of the FraiLTI study will be disseminated through VERN’s social media accounts, newsletters and dedicated webinars.
Conclusion
The FraiLTI study will provide a comprehensive overview of the prevalence of frailty, multimorbidity and sarcopenia in CLTI patients. As a UK first multicentre prospective study, we set out to provide an understanding of the scale and clinical consequences of CLTI, leading on to the development of large-scale prospective research projects on developing more focused interventions for patients with frailty and sarcopenia with the aim of improving their clinical outcomes.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(2):91-97
Publication date:
February 28, 2024
Author Affiliations:
1. The Medical School, Newcastle University, Newcastle upon Tyne, UK
2. Northern Vascular Centre, Freeman Hospital, High Heaton, Newcastle upon Tyne, UK
3. Department of Sport, Exercise and Rehabilitation, Northumbria University, Newcastle upon Tyne, UK
3. AGE Research Group, NIHR Newcastle Biomedical Research Centre, Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust, 3rd Floor Biomedical Research Building, Campus for Ageing and Vitality, Newcastle upon Tyne, UK
Corresponding author:
Sandip Nandhra
Northern Vascular Centre, Freeman Hospital, Freeman Road, High Heaton, Newcastle upon Tyne, NE7 7DN, UK
Email: [email protected]











