Systematic review
The incidence of surgical site infection following transmetatarsal amputation: a systematic review
Jess R,1 Jenkinson T,1 Al-Saadi N,1 Chetter I,2 Popplewell M,1,3 Wall ML1,3
Plain English Summary
Why we undertook the work: The rate of wound infection following a forefoot amputation is unknown. This review aimed to assess the reported rate of wound infections following a forefoot amputation. The patients in the study had complications due to diabetic foot infection or peripheral arterial disease.
What we did: Several databases were searched for studies that included patients who had undergone forefoot amputations.
What we found: The search found 298 articles, of which only five reported forefoot amputations. Through analysis, the forefoot amputation wound infection rate was 24% across 233 amputations.
What this means: Across the five relevant articles there is a high infection rate. The infection definitions, differing study methods and small number of studies make comparison difficult. To improve this, further research is needed.
Abstract
Introduction: The rate of surgical site infections (SSIs) following transmetatarsal amputation (TMA) is unknown. This study aimed to determine the reported incidence of SSIs following TMAs in patients who underwent amputation secondary to peripheral arterial disease (PAD) or complications of diabetic foot infection (DFI).
Methods: This review was conducted following the guidance outlined in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement and was prospectively registered with the international prospective register of systematic reviews. The EMBASE, MEDLINE and COCHRANE databases were searched using a pre-defined search strategy without date restriction. All randomised controlled trials (RCTs) and observational studies including patients who underwent TMA due to PAD or DFI were included.
Results: The search identified 298 articles. One RCT and four observational studies, reporting 233 TMAs were included. The overall incidence of SSI per TMA was 24.0%. There was no reporting on the severity of SSI in any of the studies. The criteria used to define SSIs were heterogeneous amongst the studies.
Conclusions: The number of studies reporting the incidence of SSIs following TMA is very small, but the SSI incidence appears high and similar to that seen following major lower limb amputation. The heterogeneity of SSI definition, differing study methodologies and the small number of studies make comparison of outcomes challenging. Further high-quality research investigating SSIs following TMA is required including assessment of specific risk factors, the impact on patient outcomes and the effectiveness of prophylactic interventions.
Introduction
Transmetatarsal amputations (TMAs) were first popularised in 1949 by McKittrick et al, who used this procedure as an alternative to more proximal amputations when addressing gangrene or infection.1 Its use has continued as an effective surgical approach in treating forefoot gangrene, infection and chronic ulceration, most commonly in patients with diabetic foot or vascular disease.2 By preserving limb length and a functioning ankle joint, it enables patients to continue to walk without the need for a prosthesis.2-4 TMAs also require lower additional energy for walking compared with more proximal amputations, increasing patient satisfaction.2-5
Wound dehiscence and skin breakdown (often due to ischaemia and small vessel diabetic disease) can require patients to return to theatre for a more proximal amputation or revision.2,6 Patients undergoing TMA tend to have a high prevalence of other risk factors (eg, obesity, malnutrition and smoking) for the development of surgical site infections (SSIs).7 Those who develop SSIs are at an increased risk of morbidity and mortality, prolonged hospitalisation and readmission or need for further surgery.2,3,7,8
The accurate incidence and factors predisposing to SSI following TMA are unclear. This systematic review aimed to determine the incidence of SSI in patients undergoing TMA for peripheral arterial disease (PAD) or diabetic foot infection (DFI).
Methods
This study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.9 The study protocol was prospectively registered with the international prospective register of systematic reviews (PROSPERO; CRD42023488553).10
Data sources, search terms and inclusion/exclusion criteria
The search strategy was developed in collaboration with a clinical librarian. The MEDLINE, EMBASE and COCHRANE databases were searched separately without date restriction using the following terms: [transmetatarsal amputation OR TMA OR forefoot amputation] AND [wound infection OR surgical wound infection OR surgical site infection]. EMBASE and MEDLINE were searched on 1 November 2023 and COCHRANE was searched on 6 November 2023.
Inclusion criteria were as follows: patients >18 years old; studies including patients undergoing TMA due to PAD or DFI; and incidence of SSI reported. Exclusion criteria were: TMA secondary to trauma or malignancy; SSI incidence not reported; case reports; and no English version of the full text available.
Review methods
Studies identified in the search strategy were exported onto Microsoft Excel and any duplicate articles were removed. Title, abstract and full-text screening was carried out by two reviewers (RJ and TJ) with any conflicts resolved by a third reviewer (either NA-S or MLW). For any studies where the incidence of SSI following TMA could not be extracted (n=1), the authors were contacted and the data requested.11
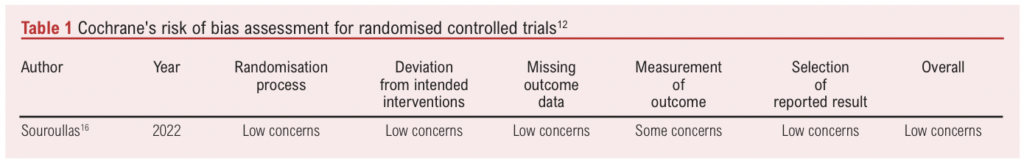
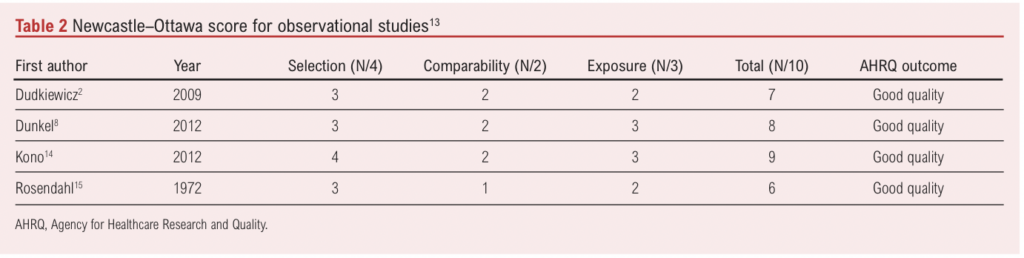
A risk of bias assessment was carried out by two independent reviewers (RJ and TJ) using the Cochrane risk of bias tool for randomised trials and the Newcastle–Ottawa Scale for observational studies (Tables 1 and 2).12,13
The primary outcome was the incidence of SSI following TMA. Data were organised and extracted onto a pre-designed data extraction tool. The incidence of SSIs was reported per TMA incision for all participants meeting the inclusion criteria. The SSI incidence in both the intervention and control arms of the RCT were included.
Secondary outcomes included: patient demographics, study follow-up period, any history of prior lower limb arterial revascularisation, the incidence of SSI according to severity, postoperative complications and length of stay.
Synthesis methods
The data collection tool was designed to allow the calculation of the incidence of SSI per TMA incision, which was carried out for all the studies. The mean was calculated for demographic variables including age and gender. Analyses were performed using Excel (Microsoft).
Results
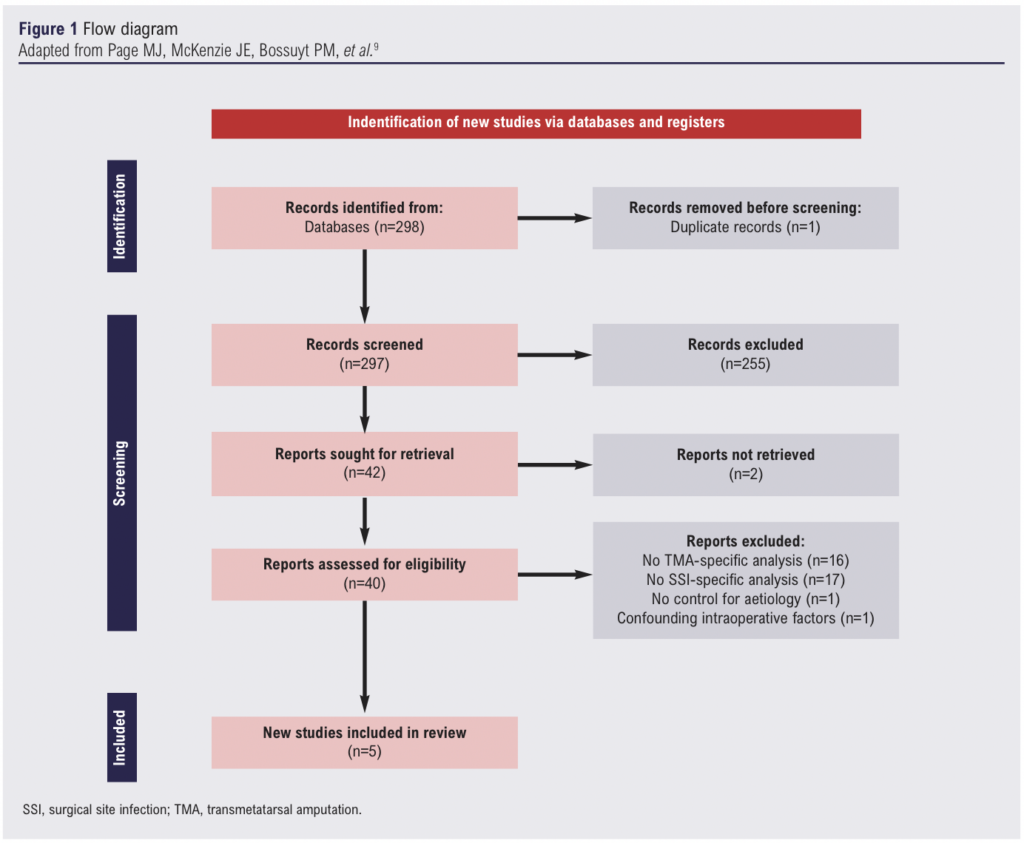
The initial search identified 298 articles. Following removal of duplicates and title screening, 96 abstracts were reviewed and 42 articles underwent full-text review. After 37 further exclusions, five articles were considered eligible and underwent full data extraction (Figure 1).
Demographic details
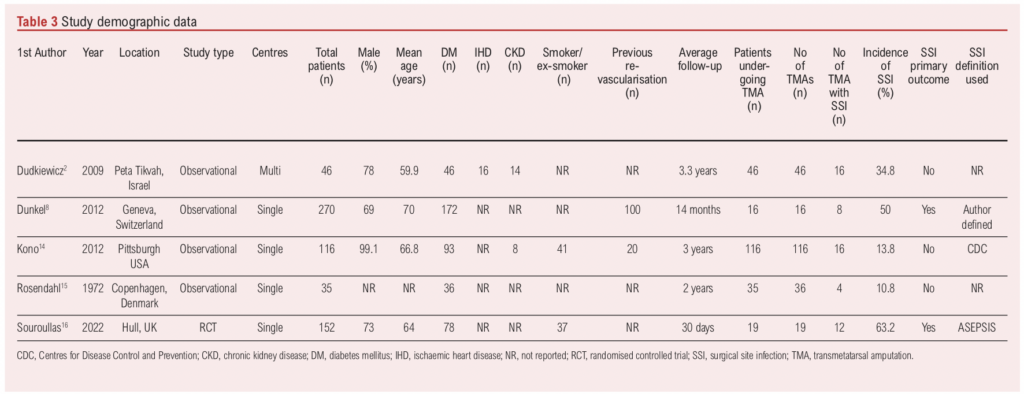
The five included studies were all published between 1972 and 2022 (Table 3).2,8,14-16 One of the studies16 was a RCT and the remaining four were observational studies. SSI was the primary outcome in two of the studies and a secondary outcome in three studies. Three of the studies used a definition for SSI which was either the Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay (ASEPSIS), Centres for Disease Control and Prevention (CDC) or an author-specified definition.17-19 The remaining two studies did not specify a definition of SSI.
Overall, 233 TMAs were performed in 232 patients. The mean age was 67 years and the mean percentage of male patients was 76.7%.
Overall SSI rates
There were 56 SSIs in 233 TMAs, equating to an SSI incidence of 24.0% per TMA No study reported the severity of SSI (superficial/deep). The SSI incidence varied considerably from 10.8% to 63.2%. In studies with SSI as the primary outcome, the SSI incidence was 57.1% (20/35), whereas in studies with SSI as a secondary outcome it was 18.1% (30/199). The incidence of SSI was highest in the RCT which used the ASEPSIS score to define SSIs.
Randomised controlled trial
The RCT with a TMA SSI incidence of 63.16% (12/19) recruited and randomised 161 individuals from a single vascular unit to receive either 5 days (intervention) or 24 hours (control) of prophylactic antibiotics.16 Of the 161 individuals, 152 were included in the final analysis (19 TMA, 89 transtibial, 8 through-knee and 36 transfemoral amputations).
A 5-day course of prophylactic antibiotics was associated with a reduction in both SSI and impaired wound healing across the whole trial. TMA was associated with an increased risk of SSI (OR 10.63, 2.82 to 40.1; p<0.001) and impaired wound healing (OR 86.89, 8.03 to 940.07; p<0.001) in those who received 24 hours of prophylactic antibiotics.16
Observational studies
The overall incidence of SSI in the observational studies was 44/215 (20.5%). There was heterogeneity in the SSI incidence between individual studies (Table 3). Notably, in the study with SSI as the primary outcome, the SSI incidence was higher (50%) than in studies with SSI as a secondary outcome.
Factors that influence SSI incidence
Patient comorbidity and surgical practice data were infrequently reported. All five studies reported the prevalence of diabetes, but data reporting regarding smoking, ischaemic heart disease, chronic kidney disease and previous revascularisation was more variable, nor was it specific to TMAs (Table 3). There also appeared to be large variability and little standardisation regarding pre-, peri- and post-operative care.
The study by Dudkiewicz et al is the only one that gave outcomes on patients who required surgical re-intervention following TMA due to postoperative wound infection, ischaemia or wound breakdown.2 In total, 21/46 patients required surgical re-intervention in the form of wound debridement or higher-level amputation. Of these patients, nine had a form of wound debridement; however, this study does not distinguish between the types of debridement. Eleven patients had TMA revisions to below-knee amputations and one was revised to a Lisfranc amputation.
Risk of bias in studies
Reviewers determined that the median Newcastle–Ottawa score for observational studies was 7.5 (range 6–9; Table 1). All studies were found to be of good quality. Using Cochrane’s risk of bias tool, the RCT was found to have a low risk of bias (Table 2).
Discussion
SSIs have recently been under the spotlight in 2021 with the publication of the National Wound Care Strategy Programme (NWCSP), which offers generic best practice guidance designed to reduce SSIs in all wound beds.20 Amputation wounds are, however, likely to pose unique challenges due to patient characteristics and the nature of the aetiology.
NHS England data reported that 1872 TMAs were undertaken in 2022.21 TMA is therefore a relatively common procedure. However, given that this review identified only five studies involving 232 participants, it is clearly an under-researched procedure. This is the first systematic review to report the pooled SSI incidence following TMA for DFI or PAD. There are extensive reports of SSI following major lower amputation, but very little specific to TMA outcomes. The SSI incidence following TMA in the five studies identified in this systematic review was 24.0%, but varied greatly between studies (from 10.8% to 63.2%). The incidence of SSI was highest in studies that included it as a primary outcome. This may result from more focused and thorough follow-up and increased accuracy in SSI reporting. However, the studies with SSI as a primary outcome also had the smallest sample sizes, with the associated inherent implications regarding the generalisability of these results to everyday practice.
The study by Rosendahl et al reported the lowest incidence of SSI (10.8%), but it had no clear definition of SSI which may have resulted in a lower detection and reporting rate of SSI.15 The studies by Kono et al and Dudkiewicz et al had designs which may have resulted in significant detection and reporting bias.2,14 It was common in our review to find SSIs grouped with other postoperative complications (particularly wound complications such as dehiscence) and small inadequately powered low-level evidence studies assessing the use of novel approaches to reduce SSI post-TMA without controls from which we could derive usable data. The SSI incidence following TMA is clearly significant and may lead to surgical revision, prolonged hospitalisation, increased costs and increased patient morbidity and mortality.2,3,16 There is clearly a significant evidence gap regarding the clinical and cost effectiveness of interventions to reduce SSI following TMA. This evidence gap should be addressed with high-quality well-designed clinical studies using appropriately designed core outcome sets.
None of the five studies categorised SSI severity, primarily due to limitations in defining SSIs. Clinically it can be difficult to classify SSI severity according to CDC criteria (superficial, deep or organ/space) without imaging (CT or MRI) or surgical exploration. Perhaps future studies should consider using a more clinically relevant severity classification.
Conclusion
We report the overall incidence of SSI in patients undergoing TMA for vascular disease at 24.0%. Due to the small number of patients, including only one RCT which was further stratified for different antibiotic use and poor reporting standards of non-randomised studies, it is difficult to draw any firm conclusions regarding the true SSI rate in this patient population. We recommend multicentre high-quality studies in patients undergoing TMA, with specific focus on SSI and its prevention, using valid, reliable and responsive outcomes which are important to patients, clinicians and healthcare services.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(4):203-208
Publication date:
August 18, 2024
Author Affiliations:
1. Black Country Vascular Networks, Russell Hall Hospital, Dudley, UK
2. Academic Vascular Surgical Unit, Hull York Medical School, University of Hull, Hull, UK
3. Institute of Applied Health Research, University of Birmingham, Birmingham, UK
Corresponding author:
Rebecca Jess
Black Country Vascular Networks, Russell Hall Hospital, Pensnett Road, Dudley, DY1 2HQ, UK
Email: [email protected]











