ORIGINAL RESEARCH
Thromboprophylaxis strategies in patients undergoing endovenous thermal ablation: a UK survey
Whittley S,1 Machin M,1,2 Burgess L,1 Onida S,1,2 Carradice D,3,4 Davies AH1,2
Plain English Summary
Why we undertook the work: Varicose veins are a common condition and are typically managed through non-invasive treatments. However, these treatments can occasionally result in the development of blood clots due to their connection with larger leg veins. To gain insight into how healthcare providers reduce the risk of blood clot formation after these procedures, we explored the preventative measures currently adopted by clinicians in the UK.
What we did: An online survey was designed and circulated via email to vascular surgeons across the UK and promoted on social media. Responses were gathered and analysed to determine the current practices and trends in blood clot prevention during local anaesthetic varicose vein procedures.
What we found: Responses were gathered from 33 unique respondents across England and Northern Ireland. All respondents reported routine use of elastic stockings immediately after these procedures. Around two-thirds of clinicians reported routine prescription of anticoagulants, with a single dose of anticoagulation being the preferred practice. One-third of clinicians reported that they do not routinely prescribe anticoagulants to these participants.
What this means: There are different ways to reduce the risk of blood clots in patients undergoing varicose vein treatment, with the most common method being the prescription of blood-thinning medication. However, it is unclear whether this approach truly benefits these patients, as there is currently no high-quality evidence to support it. To address this uncertainty, it is crucial to conduct high-quality research that can either confirm or refute the effectiveness of blood-thinning medication in these cases. If it turns out that these medications do not provide any real benefit to these patients, it could potentially lead to cost savings for the NHS and prevent patients from experiencing unnecessary side effects.
Abstract
Introduction: It remains unclear whether patients undergoing endovenous thermal ablation (EVTA) for superficial venous incompetence (SVI) should receive pharmacological thromboprophylaxis. A survey was conducted to assess current thromboprophylaxis practices across the UK in patients undergoing EVTA for SVI.
Methods: To examine the thromboprophylaxis practices of clinicians performing EVTA for SVI in the UK, an online survey was developed using the Qualtrics online survey tool. The survey link was circulated via email to members of the multidisciplinary collaborative Vascular and Endovascular Research Network (VERN) and promoted through social media. The primary focus of the survey was to gather information regarding venous thromboembolism (VTE) prophylaxis during EVTA for SVI.
Results: A total of 32 vascular surgeons and one vascular nurse specialist based in the UK participated in the survey. All respondents reported routine prescription of compression therapy in the immediate postoperative period. Of all the respondents, 67% (n=22) reported routine prescription of pharmacological thromboprophylaxis during the peri-procedural period. Extended prophylaxis was routinely offered by 15% (n=5) of all respondents. Among those who provided extended prophylaxis, the majority (80%, n=4) used low molecular weight heparin (LMWH), while 20% (n=1) opted for a direct-acting oral anticoagulant (DOAC).
Conclusion: The findings from this survey indicate that a significant proportion of patients undergoing EVTA for SVI routinely receive pharmacological thromboprophylaxis, with a single perioperative dose of LMWH being the prevailing practice. However, there is a notable lack of robust high-quality evidence to substantiate this practice. Grade A evidence is required to assess the potential benefit of pharmacological thromboprophylaxis in the context of EVTA to guide the development of clinically relevant guidelines. Should pharmacological thromboprophylaxis prove to offer no additional benefit for this specific patient population, this could result in cost savings for the NHS and enable patients to avoid unwanted side effects associated with anticoagulation therapy.
Introduction
Background
Superficial venous incompetence (SVI) is a prevalent medical condition that often leads to the development of symptomatic varicose veins, significantly impacting one’s quality of life.1 Moreover, SVI carries the potential for major complications including bleeding, ulceration and phlebitis.2 Endovenous thermal ablation (EVTA) is now the recommended first-choice management for the treatment of symptomatic varicose veins, with up to 35,000 procedures being performed annually within the NHS.3-5 When compared with conventional open surgery, endothermal techniques are often preferred due to their minimally invasive nature, faster recovery time, lower wound infection rate and reduced postoperative pain.6,7
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant cause of morbidity and mortality and has considerable societal and economic implications.8 Postoperative VTE is a known complication of EVTA; however, the incidence of this remains unclear. Administration of pharmacological thromboprophylaxis serves as a preventative measure to reduce the risk of postoperative VTE and thus many clinicians prescribe anticoagulants for EVTA either peri-procedurally or for an extended period post-procedure. According to a survey conducted in 2019, the majority (73.3%) of vascular surgeons in Ireland reported routine prescription of pharmacological thromboprophylaxis for superficial endovenous intervention.9 However, there is no high-quality evidence to support this practice and thus it remains unclear whether patients undergoing EVTA for SVI benefit from pharmacological thromboprophylaxis.
There is also a lack of consensus within the current guidelines regarding the best approach to VTE prophylaxis for EVTA. The European Society of Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines provide a Class IIa recommendation for individualised prophylaxis strategies for superficial venous intervention,4 whilst guidelines issued by the National Institute for Health and Care Excellence (NICE) advise that VTE prophylaxis is generally not required for patients undergoing varicose vein surgery if they are assessed to be low-risk for VTE and have a total anaesthesia time of <90 min.10 The paucity of robust grade A evidence and clear guideline recommendations for preventing VTE in patients undergoing EVTA for SVI has resulted in significant differences in how clinicians approach thromboprophylaxis for these patients. Often, the choice relies on clinician discretion, leading to varied practices.
Aim
A survey was designed with the objective of establishing the range of thromboprophylaxis practices in vascular surgical units across the UK for patients undergoing EVTA for SVI.
Methods
Survey development
An online eight-question survey was developed by a focus group of surgeons and a Trial Manager using Qualtrics XM survey software.11 While previous iterations of comparable surveys offered valuable insights, this survey aimed to place a greater emphasis on determining thromboprophylaxis regimens for EVTA as well as identifying the specific anticoagulants chosen for extended prophylaxis.12,13 The survey was internally piloted, iterated and user tested by researchers at Imperial College London prior to distribution.
Questionnaire structure
The survey incorporated a mix of binary choices, multiple-choice and open-ended questions, allowing respondents to provide free-text responses. The survey was split into two sections to gather data on the following aspects:
Regional Information: To ensure a comprehensive representation of the entire UK, this section was designed to gather data pertaining to the institutions where the respondents were actively involved in their respective practices.
Thromboprophylaxis regimens: This section focused on exploring the different thromboprophylaxis regimens adopted during EVTA for SVI. It encompassed questions regarding the application of both mechanical and pharmacological approaches to thromboprophylaxis.
Questionnaire distribution
The survey link, along with the survey’s objectives, was circulated via email to members of the multidisciplinary collaborative Vascular and Endovascular Research Network (VERN) and reminders were sent via email at appropriate intervals to enhance the response rate.14 The survey link was also promoted online through social media.
Questionnaire analysis
Survey responses were gathered using the Qualtrics XM platform and data were exported to Excel.
Ethics and governance
Ethical approval was not required for the study as it focused on surveying healthcare professionals and did not include patients.15 Participation in the survey served as an indication of consent, and any respondent identifiable information was treated confidentially.
Results
Respondents
A total of 37 respondents contributed to the survey. Of these 37 respondents, four were removed as they were outside the UK. A total of 33 unique and valid responses were therefore included in the analysis. Survey responses were gathered from a total of 28 vascular centres widely distributed across the UK (Figure 1). Most respondents (97%, n=32) were vascular surgeons and 3% (n=1) were vascular nurse specialists.
Provision of compression therapy
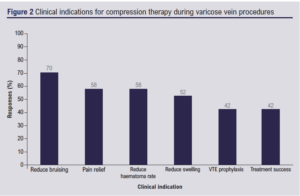
All respondents (n=33, 100%) reported routine prescription of compression therapy in the immediate postoperative period in the absence of clinical contraindications. Of the 33 respondents, 55% (n=18) routinely used either compression stockings or compression bandaging, while 39% (n=13) only offered compression stockings, and 6% (n=2) only offered compression bandaging. The reported clinical indications for using compression therapy post-procedure were to reduce bruising (n=23, 70%), pain relief (n=19, 58%), reduce haematoma rate (n=19, 58%), reduce swelling (n=17, 52%), VTE prophylaxis (n=14, 42%) and treatment success (n=14, 42%) (Figure 2). These options were presented in a multiple-choice format, allowing respondents to select more than one option.
Provision of pharmacological thromboprophylaxis
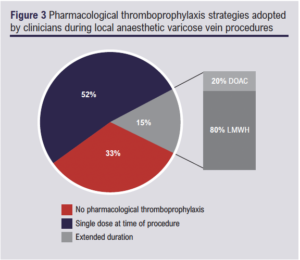
Of the 33 respondents, 33% (n=11) reported that they did not routinely prescribe pharmacological thromboprophylaxis to patients undergoing EVTA, while 67% (n=22) reported routine prescription of pharmacological thromboprophylaxis (Figure 3). Seventeen of the respondents (52%) reported that they routinely prescribed a single dose of pharmacological thromboprophylaxis at the time of procedure, while five (15%) routinely prescribed an extended course of pharmacological thromboprophylaxis. Of the five respondents who provided extended thromboprophylaxis, four (80%) routinely used low molecular weight heparin (LMWH) while one (20%) opted for a direct-acting oral anticoagulant (DOAC).
Discussion
Our findings indicate that all respondents offered at least one form of compression therapy to all patients in the immediate postoperative period following EVTA for SVI. This practice aligns with the current ESVS 2022 Clinical Practice Guidelines, which provide a level IIa, Grade A, recommendation for compression therapy after EVTA for SVI.4 Furthermore, our findings are consistent with those of a similar survey conducted in 2016, which specifically examined compression regimes following endovenous ablation for SVI.12
There is a discernible trend among clinicians who are progressively showing a preference for using compression stockings over bandages.12 This growing preference may be attributed to the convenience of applying stockings, which is particularly valuable in healthcare settings where time constraints are prevalent. Additionally, stockings are available in standardised sizes, which reduces the variability associated with operator-dependent bandage application.16 However, it has also been suggested that the choice of compression therapy following EVTA may be influenced by the selected treatment modality.12 This suggestion implies a potential shift in the endothermal techniques being performed for SVI over the last decade, possibly due to the adoption of more cost-effective techniques.17
In contrast to the ESVS guidelines, which recommend the use of compression after EVTA for SVI, the NICE guidelines present a contradicting recommendation. NICE guidelines advise that mechanical thromboprophylaxis following EVTA should only be considered for patients who are at an increased risk of VTE and where pharmacological thromboprophylaxis is contraindicated.10 Our survey, however, indicated that less than half of respondents used compression therapy for VTE prophylaxis.10 Instead, the majority of respondents reported alternative clinical indications unrelated to VTE prophylaxis, such as reduced bruising. Recent patient and public involvement work has revealed that patients are inclined to favour receiving compression therapy due to these non-thrombotic benefits. Consequently, patients who may not be at an increased risk of VTE are likely to receive postoperative compression, despite NICE guidelines suggesting otherwise.10 The observed variability in clinical indications for postoperative compression aligns with the existing literature, which recognises compression therapy as an established approach for mitigating postoperative pain following EVTA.18,19 However, the effectiveness of compression therapy in reducing swelling, improving treatment success and serving as VTE prophylaxis remains uncertain.20.21
This survey indicates that all three thromboprophylaxis practices are adopted across the UK and are considered the standard of care, with a preference for a single perioperative dose of LMWH. A consensus study conducted in 2020 also revealed that consultants predominantly prescribed a single perioperative dose of LMWH for moderate-risk VTE patients.13 This practice is consistent with the findings of a recent meta-analysis which showed a significant reduction in DVT rates following pharmacological thromboprophylaxis for endovenous varicose vein interventions.22 Our survey findings are further supported by a survey conducted at The Royal Society of Medicine Venous Forum 2023, a national vascular conference focused on venous disease treatment. In a session pertaining to SVI, attendees were surveyed to gain insights into their typical approach to VTE prophylaxis during EVTA. Out of 32 respondents, 31% did not routinely prescribe pharmacological thromboprophylaxis for these procedures, while 53% and 16% reported routine use of a single perioperative dose and up to two weeks of anticoagulation, respectively. There is, however, a notable lack of robust grade A evidence to substantiate the provision of pharmacological thromboprophylaxis in this patient population. High-quality evidence is essential to either support or refute this current practice. The upcoming THRIVE trial (UK), funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) in 2022,23 will serve as a large randomised controlled trial providing grade A evidence to inform future practices. If pharmacological thromboprophylaxis is demonstrated to offer no additional benefit to patients undergoing endovenous interventions, discontinuing this practice could result in cost savings for the NHS. Moreover, avoiding unnecessary administration of anticoagulants provides benefits to patients by minimising the potential for hindering treatment success and preventing adverse effects associated with anticoagulant use, including excessive bleeding – a concern that was highlighted in our recent research on patient perspectives.
Risk stratification prior to endovenous intervention is essential given the heterogeneity in VTE risk among individuals.22 In 2020, the Venous Forum of the Royal Society of Medicine (RSMVF) issued guidelines aimed at assisting clinicians in determining VTE prophylaxis strategies for varicose vein procedures.24 These guidelines classify patients into three risk categories: low-risk patients, for whom anticoagulation prophylaxis lacks conclusive evidence; patients at ‘additional risk’, who are likely to require extended prophylaxis; and high-risk patients, who should receive extended prophylaxis.
In 2022 the ESVS introduced a Class I recommendation for individualised risk assessment for patients undergoing superficial venous intervention, along with a Class IIa recommendation for considering individualised prophylaxis.4 However, it is important to note that this recommendation is based on two randomised controlled trials, one of which did not incorporate risk assessments while the other focused solely on moderate-risk patients.25,26 Even on a global scale, guidelines remain somewhat ambiguous. The American Venous Forum (AVF) guidelines provide a Grade 2B recommendation for selective prophylaxis post-risk assessment.27 They advise against thromboprophylaxis using LMWH, low-dose unfractionated heparin, or fondaparinux for patients without additional thromboembolic risk factors. This recommendation, however, references the American College of Physicians (ACP) guidelines,28 which do not specifically address endovenous ablation procedures. Furthermore, the ESVS and RSMVF guidelines are contradicted by the current NICE guidelines, which state that VTE prophylaxis is generally not required for patients undergoing varicose vein surgery at low risk of VTE with a total anaesthesia time of <90 min.10
In the UK, the Department of Health Risk Assessment (DHRA) tool is used to stratify patients undergoing endovenous interventions for SVI and assess their VTE risk.29 However, consultants perceive risk factors for VTE in this patient population that are not adequately captured by this tool.13 Additionally, other risk assessment models, such as the widely-used Caprini RAM in Europe and the United States for varicose vein patients, have been reported to have limited predictive accuracy for VTE.30 Hence, a validated preoperative risk assessment tool is needed to accurately stratify patients before endovenous interventions for SVI and ensure appropriate thromboprophylaxis based on their risk level.
Compared with previous reports,9,13 there appears to be a rising trend among clinicians in choosing to abstain from the administration of pharmacological thromboprophylaxis for patients undergoing EVTA for SVI. Our findings suggest that this percentage has risen to 33%, whereas it stood considerably lower at 6.7% in 2019 and 5% in 2020. This is interesting, given the introduction of the ESVS recommendation in 2022 advising clinicians to consider individualised prophylaxis following a personalised risk assessment.4 One possible explanation for this shift could be that clinicians are conducting more comprehensive risk assessments to identify patients less likely to require pharmacological thromboprophylaxis.4 Additionally, financial considerations, including budget constraints, may be influencing these decisions, as the provision of pharmacological thromboprophylaxis and the management of potential anticoagulant side effects can incur significant costs. Further investigation is needed to determine whether there are any discernible trends regarding thromboprophylaxis administration based on the healthcare setting (NHS vs private).
Determining the true incidence of postoperative VTE in patients undergoing EVTA for SVI presents a formidable challenge, primarily due to the high heterogeneity observed in current study designs,22 as noted in the literature. Consequently, there is currently a lack of consensus on this matter. While prevailing estimates place the incidence within a range of 0.51–3.2%,31,32 some reports have even suggested an incidence as low as 0%.33 Determining the true incidence of VTE in this patient population holds significant importance as it would not only facilitate the development of evidence-based guidelines that can adequately inform thromboprophylaxis practice but would also aid clinicians in assessing the overall risk associated with endovenous procedures and make informed decisions regarding VTE prophylaxis. The forthcoming THRIVE trial is also expected to contribute to addressing this important issue.23
Limitations
Although this survey gathered responses from various vascular centres across England and Northern Ireland, it is important to note the absence of responses from Scotland and Wales. Therefore, while this survey provides insights into the prevailing thromboprophylaxis practices in England and Northern Ireland, its ability to accurately reflect practices in Scotland and Wales remains questionable, possibly restricting the generalisability of the findings. It may be valuable to consider conducting a future iteration of this survey, with a focus on reaching out directly to specific regions in the UK and Ireland via email to enhance their representation. The relatively small overall sample size and reliance on self-reported data may also lead to selection bias, potentially further impacting the generalisability of the findings.
Conclusions
The results of this survey suggest that, in the UK, the prevailing practice for thromboprophylaxis following EVTA for SVI is a single perioperative dose of LMWH. There is, however, a range of practices in this regard, underpinned by a lack of clear high-quality evidence-based guidelines. Grade A evidence is therefore required to evaluate the potential benefits of pharmacological thromboprophylaxis within this specific patient population, thereby either validating or refuting the current practices. Future research endeavours could involve conducting a similar survey to track the evolving trends in practice over time. Additionally, investigating whether disparities in practices exist between the NHS and the private sector would be valuable, aiming to discern whether the healthcare setting itself influences thromboprophylaxis practices.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2023; 3(1): 26-31
Publication date:
November 14, 2023
Author Affiliations:
1. Academic Section of Vascular Surgery, Department of Surgery and Cancer, Imperial College London, Charing Cross Hospital, London, UK
2. Imperial Vascular Unit, Imperial College Healthcare NHS Trust, St Mary’s Hospital, London, UK
3. Academic Vascular Surgery Unit, Hull York Medical School, Hull, UK
4. Department of Vascular Surgery, Hull University Teaching Hospitals NHS Trust, Hull, UK
Corresponding author:
Alun Huw Davies
Professor of Vascular Surgery, Department of Surgery and Cancer, Imperial College London, London W6 8RF, UK
Email: [email protected]











