COHORT STUDY
SHOCkwave lithotripsy for patients with peripheral arterial disease: the SHOCC study
Lopez-Pena G,1* Musto L,1* Finch SL,2 Bown MJ,2 Davies R,1 Sohrabi S,4 Rees O,3 Lakshminarayan R,4 Saratzis A,1,2 on behalf of the SHOCC Investigators4
Plain English Summary
Why we undertook the work: Blockages and narrowing in arteries supplying blood to the legs are common. This condition is called peripheral arterial disease or PAD in short. Often, these narrowings and blockages have lots of calcium. A new device called lithotripsy has been developed recently to allow healthcare professionals open up calcified artery blockages. This work aimed to look at whether lithotripsy used for PAD in the NHS is safe and leads to acceptable results.
What we did: A national research study was done, across eight NHS hospitals. A total of 91 people took part in this research, who had PAD and needed lithotripsy treatment.
What we found: Lithotripsy is safe to use as 100% of the procedures for the participants in the study were successfully completed. Complications were not more common compared to other technologies used for such calcified arteries. Also, with imaging before and after lithotripsy treatment, we found that lithotripsy breaks down artery calcium.
What this means: Lithotripsy can be used safely in the NHS. Future work should include a randomised trial, where people with PAD are treated with lithotripsy and compared to other modes of treatment.
Abstract
Objective: To assess the 6-month patency and clinical outcomes after endovascular treatment of lower limb atherosclerotic lesions in patients with peripheral arterial disease presenting with severe claudication or chronic limb threatening ischaemia (CLTI) using intravascular lithotripsy (IVL).
Methods: A prospective multicentre cohort study was carried out in eight centres in the UK. Consecutive patients with CLTI or lifestyle-limiting claudication treated with IVL as a primary vessel preparation based on operator’s preference were included. Follow-up occurred at discharge, 30 days and 6 months with clinical and duplex assessments. A subset of patients underwent computed tomographic angiographic (CTA) lesion analysis pre-/post-IVL to assess plaque consistency/morphology using three-dimensional reconstruction of the CTA.
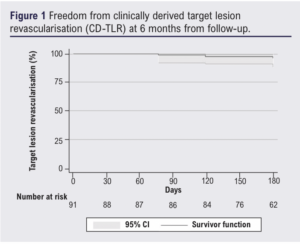
Results: Overall, 91 patients (mean age 73 years, 72% male; 81% with CLTI) were enrolled in the study between September 2021 and March 2023 (21 took part in the CTA plaque imaging substudy). All patients with claudication had presented with severe lifestyle-limiting claudication and had calf claudication at <20 metres; those with CLTI all presented with Rutherford stage 5 or 6 disease. Immediate procedural success was 100%; 15 (16%) underwent a hybrid intervention. Mean target lesion peripheral artery calcification scoring system (PACCS) grade was 3.31±1.01. At the latest available follow-up (mean 183±75 days) there were 10 deaths (11%), one access complication (1%), two major cardiovascular events (2%) and eight major amputations (9%). A total of 22 re-interventions (24%) and freedom from clinically derived target lesion revascularisation at 6 months was 96% (95% CI 0.88% to 0.99%). Automated 3D plaque subgroup analysis (n=21) revealed >50% remodelling in calcium load within the plaque in 15 plaques (71%), only two dissections (9.5%) and a significant decrease in median plaque calcium load volume (from 7.8 cm3 preoperatively to 5.4 cm3 postoperatively).
Conclusion: IVL is a safe and efficacious option for vessel preparation with high procedural success rates, acceptable medium-term complications and freedom from re-intervention.
Introduction
Peripheral artery disease (PAD) represents a major health problem worldwide, affecting one-fifth of people over the age of 60 in the UK.1–3 It is the most common cause of lower limb amputation.4
Most patients with chronic limb threatening ischaemia (CLTI) and some patients with severe claudication will require lower limb revascularisation, one of the commonest vascular procedures in contemporary vascular practice, typically in the form of endovascular reconstruction. Arterial wall calcification represents a major challenge for endovascular arterial revascularisation, often resulting in unsuccessful recanalisation, dissection or early restenosis.5-8 Intravascular lithotripsy (IVL) has emerged as a vessel/lesion preparation strategy to help overcome these issues.9 The IVL device shatters the calcium within the atherosclerotic plaque using ultrasound (energy) via an angioplasty balloon. A recently completed randomised controlled trial (DISRUPT PAD III) involving 306 lesions (femoropopliteal stenoses or occlusions) treated with either IVL and angioplasty or conventional means (no IVL) showed that IVL resulted in greater procedural success, fewer dissections and comparable rates of major adverse events at 30 days.10 The DISRUPT PAD III randomised controlled trial presented 2-year patency outcomes in a primarily claudicant population;10,11 however, it was powered on intra-procedural events and included one vessel bed. There is also a paucity of high-quality prospective multicentre clinical data regarding the use of this technology, especially in patients with CLTI. This multicentre prospective study aimed to assess clinical and imaging outcomes following the use of IVL in a population consisting mostly of patients with CLTI across all lower limb vessel beds over a minimum 6-month structured follow-up period. An additional subgroup underwent computed tomographic (CT) evaluation of their target lesion pre-/post-IVL to objectively assess change in plaque morphology and consistency.
Methods
Regulatory approvals
The SHOCC study was a prospective multicentre observational study performed across eight NHS hospitals in England and Wales over an 18-month period between September 2021 and March 2023. The study received ethical and all relevant regulatory approvals (20/09/2021, Wales Research Ethics Committee (REC)) prior to commencing recruitment. The study was prospectively registered on the ISRCTN registry (ID: 76218607 – protocol available online prior to commencing recruitment) and sponsored independently by the University of Leicester. Research was funded by Shockwave Medical Inc (Santa Clara, California, USA), who had no input into the design, analysis, reporting and no access to data. All participants provided written informed consent before enrolment in the study. A study steering committee and data safety monitoring committee oversaw running of the study.
Eligibility criteria
Patients were eligible if they were >18 years of age diagnosed with CLTI or lifestyle-limiting (defined as severe) intermittent claudication and had at least one peripheral lesion deemed to require use of IVL by the treating clinician, regardless of location. Those who presented with acute disease (symptoms of <3 days duration) requiring emergency treatment or had asymptomatic PAD were excluded. Participants were recruited prior to intervention. The decision to use IVL was made by the operators based on preoperative imaging and local protocols (pragmatic study design).
Data collection and handling
Data were collected prospectively using a predefined form and via a REDCap (Research Electronic Data Capture) database hosted at the National Institute for Health Research Leicester Biomedical Research Centre.12,13 The following were collected prospectively at baseline, discharge, 30 days and 6 months: demographics, comorbidities, procedure details, all available cross-sectional imaging, cause of death, re-admissions/re-interventions, cardiovascular events; missing data were queried prospectively with recruiting sites. All patients underwent an arterial duplex scan at baseline and 6 months. Those presenting with symptoms at any point during follow-up also underwent cross-sectional imaging based on local protocols and clinician preference. A subgroup consented to have a preoperative and postoperative (within 72 hours of the procedure) CT angiogram of the lesion (focused scan) to analyse the morphology of the plaque and report calcium content using a technique we previously developed and validated, described in detail elsewhere.14–16 This automated three-dimensional (3D) plaque analysis was performed on this group using a dedicated TeraRecon 3D workstation (Aquarius iNtuition Viewer, Aquarius, TeraRecon, San Matteo, California, USA) by two independent investigators.
Outcomes and definitions
The primary outcome of interest was patency of the lesion treated with IVL at 6 months. Secondary outcomes included death, amputation, re-intervention/re-admission and cardiovascular events. Patency was defined as freedom from clinically driven target lesion revascularisation (CD-TLR) and freedom from occlusion or >50% on any cross-sectional imaging. Technical success was reported and defined as the ability to cross the lesion with both a wire and an IVL balloon and successfully use IVL as planned (per instructions for use) without immediate embolisation, occlusion or dissection. Procedural success was defined as residual target lesion angiographic stenosis <50% following use of IVL and subsequent adjunct (where necessary) without peripheral embolisation, dissection and in-line flow to the foot (minimum of one crural artery). This research was designed as a prospective cohort study aiming to capture all consecutive patients meeting the eligibility criteria at each site; no minimum sample size calculation was required.
Analysis was performed using STATA Statistical Software (Texas, USA).17 Results are reported as mean and standard deviation (SD) for normally distributed variables and median with interquartile range (IQR) for non-parametric variables. Categorical variables are reported as counts and proportion (%). Standard tests were used to assess differences between categorical and non-categorical variables of interest. A Kaplan–Meier analysis was performed to assess the primary outcome. The funder was not involved in analyses.
Results
A total of 94 patients were enrolled (70% of eligible participants across sites); three withdrew consent prior to intervention, so 91 patients took part in the study and are presented in this analysis. The baseline characteristics of the 91 patients undergoing IVL and included in the study are reported in Table 1. The mean age of the participants was 73 years (range 50–96) and 72% were male. Overall, 13% reported symptoms in both limbs, 20% presented with claudication, 25% with rest pain, 34% with tissue loss and 21% with both rest pain and tissue loss. All patients with claudication had presented with severe lifestyle-limiting claudication and had calf claudication at <20 metres; those with CLTI all presented with Rutherford stage 5 or 6 disease). The mean±SD ankle brachial pressure index at baseline was 0.21±0.03 for those with CLTI and 0.30±0.05 for those with claudication. The majority of patients (Table 2) were treated for infra-inguinal disease. Interestingly, 18 target lesions (IVL-treated) were common femoral arteries. Furthermore, 41 (45%) IVL-treated target lesions with a mean length of 10 mm were included.
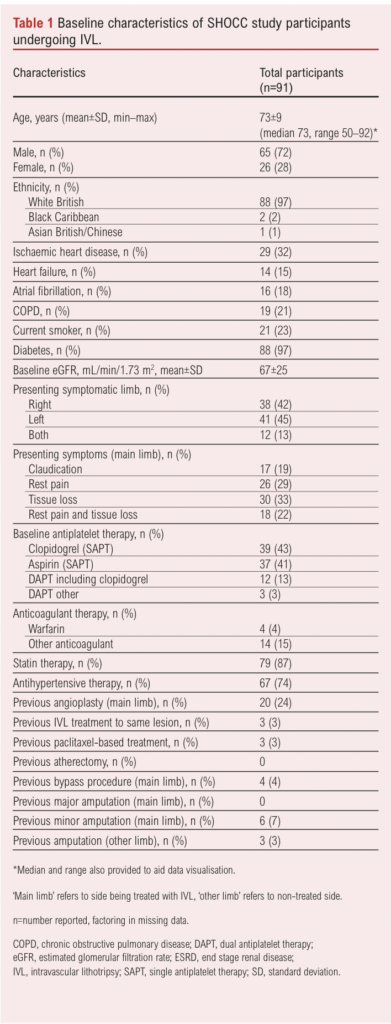
All participants were on antiplatelet therapy at baseline, 24% had undergone previous angioplasty, 4% a bypass and 7% a minor amputation to the same limb (no major amputations). Table 2 describes the procedural details and the characteristics of the atherosclerotic lesions within the treated (symptomatic) limb of the patients. Most were femoro-popliteal – that is, superficial femoral artery (SFA) or popliteal (34% proximal SFA, 43% mid SFA, 63% distal SFA, 45% P1, 35% P2, 26% P3) lesions. The mean peripheral artery calcification scoring system (PACSS) grade of the target lesions was 3.31.8 Overall, 16% underwent hybrid procedures (combined open and endovascular – all common femoral endarterectomies in the case of open procedures); adjuncts to IVL included plain balloon angioplasty (50%), drug-coated balloons (23%, all paclitaxel) and drug-eluding stents (9%, all paclitaxel). Most procedures were performed under locoregional anaesthesia (71%) rather than sedation (7%) or general anaesthesia (22%). The ipsilateral common femoral artery was the most common access vessel (92%) while the brachial artery (3%) and superficial femoral artery (3%) also featured. Technical success was 100% and procedural success was 71% (no residual stenosis of the target lesion following IVL and adjunct with in-line flow to the foot on completion angiogram, and no dissections or peripheral emboli – all ‘procedural success’ failures were secondary to residual target lesion stenoses of 50–70% on the completion angiogram without flow-limiting dissections or emboli detected). Median IVL catheter size was 6 mm (range 3–8 mm), used in 27% of cases; the median number of IVL cycles was six.
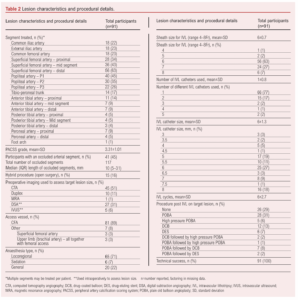
Table 3 shows outcomes over 6 months. Median length of stay was 3 days (range 0–88 days). Re-intervention occurred in 24% of cases, with a similar volume of endovascular (13%) and open (11%) re-interventions. All open re-interventions were common femoral artery endarterectomies and femoro-popliteal bypasses (with venous conduit). All endovascular re-interventions were plain balloon angioplasties. The majority of these were not specifically for the IVL-treated lesions, intervention for IVL-treated lesions occurring in 10% (9 patients, 7% by endovascular means and 3% by open surgery – all common femoral endarterectomies). Target lesion re-stenosis >50% within 6 months occurred in 19 patients at their latest follow-up and none were symptomatic with stenosis of the lesion that was originally treated using IVL. The Kaplan–Meier estimate for freedom from CD-TLR was 96% (95% CI 0.88% to 0.99%) at the end of follow-up (Figure 1). Overall, 11% of patients died during the follow-up period, mostly due to heart failure (3%) and sepsis (3%). Major amputations occurred in 9% of the cohort and minor amputations in 12%; one patient initially underwent a minor amputation and then a major amputation procedure within 6 months. Major cardiovascular events occurred in two patients (2%), both of whom underwent urgent coronary revascularisation procedures. Other complications included a single access complication (1%), one intensive care admission due to severe delirium post procedure, and another for marked hyperkalaemia. There were no episodes of acute limb ischemia during the follow-up period. No dissections (outside of the plaque analysis subgroup below) were reported.
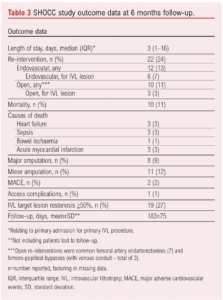
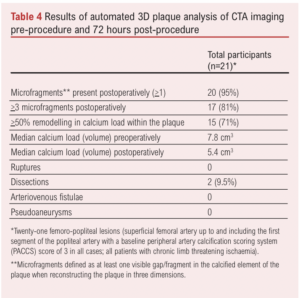
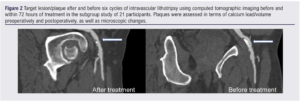
A subgroup of patients (n=21) underwent automated 3D plaque analysis, the results of which are shown in Table 4 and Figure 2. The inter-observer correlation (20 CTAs analysed by two investigators) was 0.93 (95% CI 0.88 to 0.96) and intra-observer correlation (20 CTAs analysed by the same observer twice) was 0.95 (95% CI 0.85 to 0.99) Lesion length exceed 10 cm in all cases and all were classified as PACCS 2 preoperatively (femoro-popliteal lesions in all cases). All 21 procedures were completed successfully and patients were only treated with balloon angioplasty after application of IVL (three cycles of IVL as per instructions for use in all lesions). Microfragments were present in the majority of treated lesions (95%) with 81% of plaques containing >3 microfragments (visible gap in the calcified segment of the plaque on 3D reconstruction at maximum zoom). Overall, 71% had a 50% remodelling in calcium load within the plaque, demonstrated by a reduction in median calcium load from 7.8 cm3 preoperatively to 5.4 cm3 postoperatively (calcium remodelling). No ruptures, arteriovenous fistulae or pseudoaneurysms were detected. However, two dissections were seen on plaque reconstruction (9.5%) which were not detected at the time of the procedure; neither dissection involved more than 10% of the length of the lesion and/or more than 10% of the lumen diameter.
Discussion
This is a prospective cohort study including all-comers predominantly with CLTI and highly-calcified lesions treated with IVL across several centres, showing high success rates and acceptable complication/re-intervention rates over a 6-month structured period. Most patients eligible for inclusion at all recruiting sites agreed to take part (70%) and 91 participants were included in the analyses, having provided high-quality prospective follow-up data (with no missing data). This work adds pragmatic generalisable evidence regarding the use of IVL in the treatment of steno-occlusive PAD as a means of vessel/lesion preparation when treating calcified plaques. The imaging subanalysis also provides novel data regarding the microscopic effects of IVL in calcified lesions.
The main findings of the study can be summarised as follows: (1) IVL as a vessel preparation strategy is a safe procedure in challenging lesions/patients; minimal access (1%), thrombotic or cardiovascular (2%) complications were reported at 30 days, 90 days and 6 months. (2) The femoro-popliteal segment is the most common arterial site treated, as seen in the PAD III randomised controlled trial.11,18 (3) IVL use was associated with a high technical success rate (100%), acceptable freedom from target lesion revascularisation and number of major limb amputations in this high-risk population (predominantly CLTI, all with extremely calcified lesions and multilevel disease), compared with data from recent trials and data reported in the latest guidance relating to CLTI management in Europe/globally.11,18-22
Previously, in the PAD III trial, IVL was shown to be superior to percutaneous transluminal angioplasty (PTA) in terms of procedural success. Vessel preparation with IVL was safely performed using a significantly lower maximum inflation pressure relative to PTA, resulting in lower rates of dissection and a lower risk of post-dilatation and provisional stent placement. The trial concluded that IVL may be a valuable tool for treating patients with calcified arterial lesions, potentially leading to improved outcomes and reduced complications in the management of PAD with heavily calcified arteries.18
In a real-world setting, the use of peripheral IVL demonstrated low residual stenosis, high acute gain and a low rate of complications despite the complexity of the disease. Specifically, the average acute gain was 3.4 mm at the end of the procedure with a final mean residual stenosis of 23.6%. Additionally, angiographic complications were rare, with a single perforation following drug-coated balloon inflation unrelated to the IVL procedure out of the 114 femoro-popliteal lesions analysed in the observational real-world trial PAD III.11
This study has replicated the findings of the previously conducted efficacy-driven IVL trials in a pragmatic CLTI (mostly) setting, showing a similar safety profile, minimal angiographic complications, high technical (100%) and procedural success (71%), no clinically significant dissections or perforations and few access complications. During follow-up only 19 patients had restenosis of the target lesion >50%. It is unknown if the restenosis is related to using IVL, natural progression of the disease, or other adjunctive procedures that were conducted such as stenting. However, in the subgroup analysis of the patients who had an additional CT scan we found no evidence of major complications at the microscopic level due to the use of IVL.
Study limitations
This study has several limitations. First, it is an observational study without a control group, which limits the ability to make direct comparisons with other treatment modalities. Additionally, maximal follow-up was 6 months, therefore the long-term durability of the IVL treatment was not assessed. Furthermore, the study included treatment in multiple vascular segments, which makes it difficult to apply the results to a specific PAD-affected segment.
Conclusion
These findings have important implications for the treatment of PAD in patients with heavily calcified arteries, as they suggest that IVL is a safe and efficacious option for vessel preparation in this patient population involving mostly patients with CLTI and some with severe claudication. Longer-term follow-up is required to fully understand the impact of these short to mid-term outcomes on longer-term results. Additionally, further research is needed to compare IVL with other treatment modalities such as atherectomy (ie, other vessel preparation strategies), conducting formal cost analyses to fully understand the impact of vessel preparation in this context.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(3):140-146
Publication date:
May 22, 2024
Author Affiliations:
*Joint lead authors
**SHOCC Investigators’ list available in Appendix 1 online atwww.jvsgbi.com
1. Leicester Vascular Institute, Glenfield Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK
2. Department of Cardiovascular Sciences and NIHR Leicester Biomedical Research Centre, University of Leicester, Glenfield Hospital, Leicester, UK
3. Glan Clwyd Hospital, Betsi Cadwaladr University Health Board, Bodelwyddan, Rhyl, UK
4. Hull Royal Infirmary, Hull, UK
Corresponding author:
Athanasios Saratzis
Department of Cardiovascular Sciences, University of Leicester, Leicester LE3 9QP, UK
Email: [email protected]











