COHORT STUDY
Single-centre prospective cohort study investigating the associations and one-year trends of frailty, cognition, disability and quality of life pre- and post-intervention for chronic limb-threatening ischaemia
Houghton JSM,1,2,3 Essop-Adam A,1,3 Nduwayo S,1,2,3 Nickinson ATO,1,2,3 Black I,1,3 Bryant N,1,3 Meffen A,1,4 Gray LJ,4 Conroy SP,5 Payne TJ,1,3 Rayt HS,1,2 Sayers RD,1,2,3 Haunton VJ1
Plain English Summary
Why we undertook the work: We studied the effects of frailty in people with severe artery disease in the legs who had surgery. Frailty is the decline in the body’s systems due to getting older. People with frailty get better more slowly after illness or surgery. They are more likely to need care and have a shorter life than those who don’t have frailty. About half of people with severe artery disease in the legs also have frailty. Their symptoms can limit their walking, causing disability. Long-term foot pain, ulcer or gangrene can also impact other body systems. We know that people with both severe artery disease of the legs and frailty have a shorter life and more problems after surgery. But, many still need surgery to save their leg. We compared people with and without frailty who had surgery for severe artery disease of the legs. We looked at quality of life, mood and disability over 1 year after surgery. We felt this work was important for helping patients and their doctors with severe artery disease in the legs make decisions about their care.
What we did: Ninety-nine people with severe artery disease in the legs agreed to take part. They had tests of frailty, their memory and thinking, and their physical function. They also answered surveys about quality of life, disability and mood. Eighty-seven people had a procedure. These people also took surveys 3 months and 1 year after their surgery.
What we found: Our results show that people with frailty had worse quality of life, mood and disability before and 1 year after their surgery. However, both people with and without frailty had an average improvement in mood and quality of life. On average, all patients became slightly more disabled over 1 year. About one in 10 people with frailty improved so much after surgery that they were not frail at 1 year. These people were younger. But, nearly a quarter of the people without frailty before their surgery became frail over 1 year. These people were slightly older.
What this means: Most people in this study had a better quality of life at 1 year, even those with frailty before their procedure. This means even people with frailty may benefit from a procedure for severe artery disease in the legs. These results may help people thinking about a procedure for severe artery disease in the legs to predict what their likely outcomes might be and manage expectations. These results will also help their clinicians provide crucial information for shared decision making.
Abstract
Background: Half of people with chronic limb-threatening ischaemia (CLTI) have frailty. This study aimed to describe the associations of frailty with cognition, disability and quality of life (QoL) among CLTI patients over 1 year following surgical or endovascular procedures.
Methods: A single-centre prospective cohort study was undertaken. Patients undergoing a procedure for CLTI between May 2019 and May 2021 were eligible (minimum age >65 initially; >50 from November 2019). Participants underwent preoperative assessments for frailty, physical and cognitive function, disability, mood, disease-specific QoL (Vascular QoL questionnaire (VascuQoL)) and generic health-related QoL (EuroQoL EQ-5D-5L). Follow-up was at 3 months (clinic or telephone) and 12 months (telephone). Baseline frailty was assessed using both the Edmonton frail scale (EFS) and the clinical frailty scale (CFS). Frailty during follow-up was re-assessed at 3 and 12 months using the CFS as it can be performed via telephone. Associations of baseline frailty with disability, QoL and mood scores during follow-up were investigated using repeated measures mixed models.
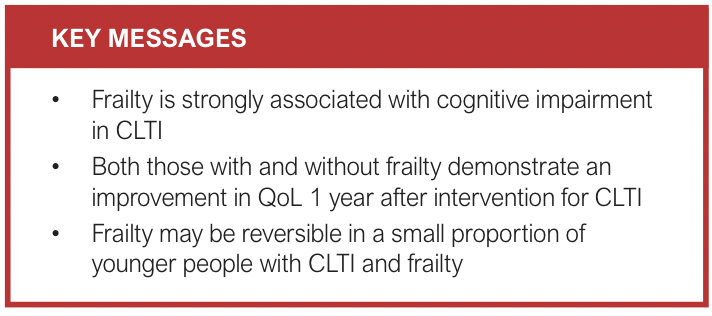
Results: Ninety-nine patients completed the baseline assessments. Forty-five (45%) were classified as frail by the EFS. Frailty was associated with a higher prevalence of cognitive impairment based on the Montreal cognitive assessment (52% vs 17%; p<0.001). Eighty-seven patients were eligible for follow-up. Baseline frailty (EFS) was associated with worse QoL scores at all timepoints (VascuQoL p=0.001; EQ-5D-5L p<0.001). Both those with and without frailty at baseline (EFS) had modest improvement in QoL scores at 12 months (VascuQoL p<0.001; EQ-5D-5L p=0.001). Barthel index (disability) scores were lower for those with frailty at baseline (EFS) (p<0.001) and decreased slightly over 12 months for both groups (p=0.007). Five patients (12%) transitioned from frailty to non-frailty at 12 months based on the CFS. However, 10 patients (23%) transitioned from non-frailty to frailty.
Conclusions: CLTI patients with frailty have worse QoL and greater disability both pre- and post-intervention. However, they demonstrate similar QoL benefit to those without frailty at 1 year following intervention. Baseline frailty assessment is important to inform prognostic discussions, expectations and shared decision making in CLTI.
Introduction
Frailty is a complex, dynamic, multi-system health state characterised by susceptibility to significant homeostatic dysregulation from even minor physiological stressors, leading to poor health-related outcomes such as loss of independence and death.1,2 Frailty is present in around half of all individuals with chronic limb-threatening ischaemia (CLTI) and is related to severity of disease.3-5 Among vascular surgery patients, including those with CLTI, frailty is related to worse perioperative outcomes and long-term survival.3-7
Frailty is closely interrelated to disability and cognitive impairment.2,8 There is a high prevalence of cognitive impairment among vascular surgery patients, but it is unknown among those with CLTI specifically.9 CLTI causes lower limb dysfunction and associated disability; however, their interactions with frailty in those with CLTI have not been described.3,10,11 Furthermore, whilst transition in frailty states has been detailed among vascular surgery patients in general, there has been little investigation of frailty trajectories following intervention for CLTI.12
The aim of this study was to describe the associations of frailty with cognition, disability, physical function, mood and quality of life (QoL) among individuals with CLTI at initial presentation and over a 1-year period following intervention.
Methods
The Leg Ischaemia Management collaboration (LIMb) study is a single-centre, prospective cohort study of individuals with CLTI (NCT04027244). The full LIMb study protocol has been published elsewhere.13 Adults with CLTI presenting to the Leicester Vascular Institute (Glenfield Hospital, University Hospitals of Leicester NHS Trust) were eligible for inclusion. CLTI was defined as a minimum 2-week ischaemic night or rest pain and/or ulceration or gangrene in the affected leg(s) attributable to confirmed peripheral artery disease.14,15 Recruitment opened in May 2019 and continued until March 2022. Written informed individual patient consent was gained prior to completion of any study procedures. Patients lacking mental capacity to consent were eligible for inclusion with agreement from a suitable personal consultee. The LIMb study received ethical approval from the UK National Research Ethics Service (19/LO/0132).
Frailty and cognitive additional assessments
Those recruited to the LIMb study aged >65 years who planned to undergo a procedure for CLTI were eligible to consent to additional frailty and cognitive assessments prior to their intervention and at 3 and 12 months post-procedure. Minimum age for inclusion was lowered to >50 years following a protocol amendment in November 2019 after identification, early in the study, of several individuals recruited to the LIMb primary cohort who were living with frailty (clinical frailty scale (CFS) score >5) but aged 50–64 and therefore initially ineligible to participate in the frailty and cognitive additional assessments.
Baseline demographics, comorbidities, preoperative blood test results and wound, ischaemia, and foot infection (WIfI) scores were collected for all patients recruited to the LIMb study. WIfI scores were converted to clinical stage based on risk of major amputation.16 Charlson Comorbidity Index (CCI) scores were calculated using an updated weighting.17 CFS, Barthel index of activities of daily living, Hospital Anxiety and Depression Scale (HADS) and the Vascular Quality of Life (VascuQoL) 25-item questionnaire were also collected at baseline.18-21 The EuroQoL EQ-5D-5L tool was added to the study schedule after a protocol amendment in May 2020.22 EQ-5D-5L scores were converted to validated values for UK patients prior to analysis.23 Individuals lacking mental capacity to consent were supported in completion of QoL questionnaires by their personal consultee if required.
Those consenting for additional frailty and cognitive assessments undertook the Edmonton frail scale (EFS), Montreal cognitive assessment (MoCA), Short Physical Performance Battery (SPPB) and standardised bilateral seated hand grip strength (Jamar Plus+ digital hand dynamometer). The original study protocol scheduled follow-up clinic visits at 3 and 12 months where frailty and cognitive assessments were repeated. A summary of assessments is shown in Table 1. Baseline frailty and cognitive assessments used in this study are not part of routine clinical practice (except for the CFS) and were not used by the clinical team in decision making.
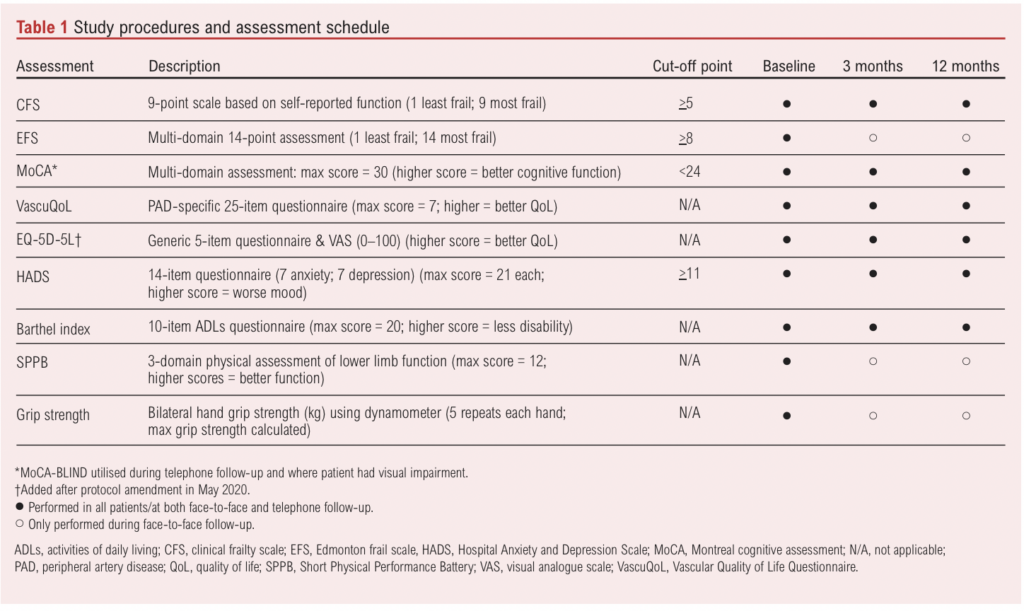
Outcome data were collected from written and/or electronic medical records. Study data were collected and managed using REDCap (Research Electronic Data Capture).24
Protocol amendments due to COVID-19
The LIMb study was paused in March 2020 at the beginning of the first UK wave of the coronavirus disease 2019 (COVID-19) pandemic. Recruitment to the frailty and cognitive additional assessments reopened in October 2020 but was again suspended throughout January 2021 during a further UK wave of COVID-19. Whilst recruitment to the primary cohort of the LIMb study was extended until March 2022, it was not possible to extend recruitment to the frailty and cognitive additional assessments beyond the original planned study end date of May 2021.
Because of COVID-19 related restrictions, all 3-month and 12-month follow-ups were undertaken via telephone from March 2020. Patients recruited to the study from February 2021 were offered the option of clinic follow-up at 3 months post-procedure. All 12-month follow-up was switched to telephone only. The MoCA-BLIND was used to assess cognitive function via telephone, but it is not possible to perform the EFS, SPPB or hand grip strength via telephone (Table 1).25
Statistical analysis
As EFS is a multi-domain clinical assessment of frailty, the baseline EFS score was used to define frailty in the analyses.26 Variables were presented in tables with data for frail (EFS >8) and non-frail (EFS <8) patients separately. Categorical variables were presented as frequencies (%). Histograms were assessed for normality of continuous variables. Normally distributed data were presented as means (standard deviation (SD)) and skewed data as medians (interquartile range (IQR)). Associations of baseline variables with frailty were investigated using a χ2 test for categorical data, t-test for normally distributed continuous data, and Kruskal–Wallis test for skewed continuous data. Cohen’s κ was calculated to assess agreement between EFS and CFS.
The change in SPPB and grip strength at 3 months was investigated using analysis of covariance. Changes in 12-month assessment scores were investigated using repeated measures mixed models (restricted maximum likelihood) and adjusted predictions presented graphically by frailty status with 95% CI. Sensitivity analyses were performed by excluding those with missing data (complete case analysis).
Association of frailty with 1-year survival was presented using Kaplan–Meier survival curves, using the log-rank test to test differences between groups. Independent associations of frailty, cognitive impairment, age and CCI score with 1-year mortality (pre-selected variables) were investigated using Cox regression and reported as hazard ratios (HR) with 95% CI. Independent associations of frailty, cognitive impairment, age, CCI score and WIfI stage (pre-selected variables) with major amputation were tested using Fine-Gray competing risk analysis (death as the competing risk) and reported as sub-distribution hazard ratios (SHR) with 95% CI.
All statistical analyses were performed in Stata (version 17 for Windows, StataCorp. College Station, Texas, USA). A p value of <0.05 was considered statistically significant.
Results
Baseline associations with frailty
In total, 99 (63%) of the 158 eligible patients recruited to the LIMb study completed additional frailty and cognitive assessments at baseline (Figure 1). Four of these patients (4%) were included via personal consultee assent (lacked mental capacity). Forty-five patients (45%) were classified as frail by the EFS and 49 (49%) by the CFS with moderate agreement (78%; κ=0.56). Breakdown of severity of frailty was similar for both EFS and CFS; however, only 16% of patients were classified as vulnerable by the EFS compared with 42% by the CFS (Table 2). Overall, 32 patients (33%) were deemed to have cognitive impairment. Cognitive impairment (MoCA <24) was strongly associated with frailty (52% vs 17%; p<0.001).
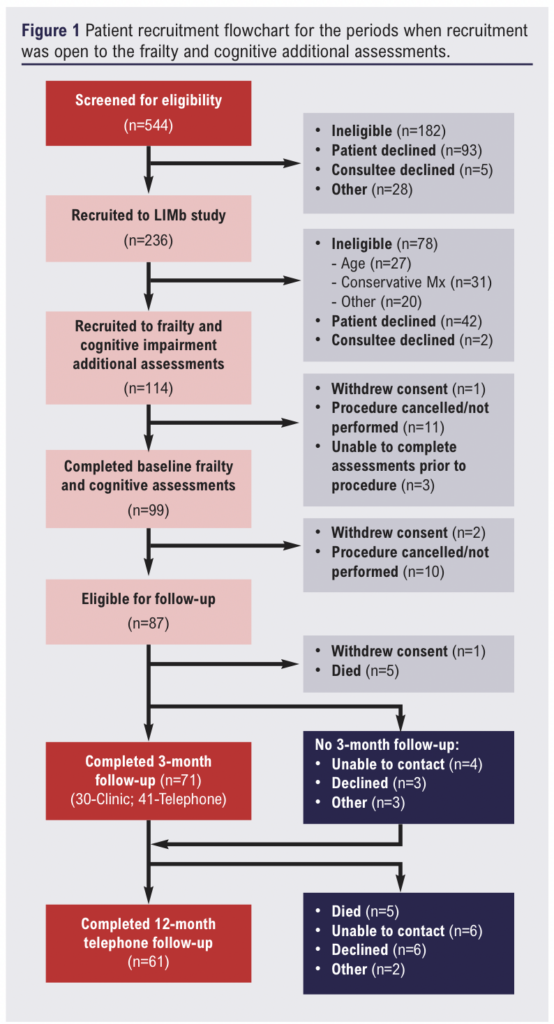
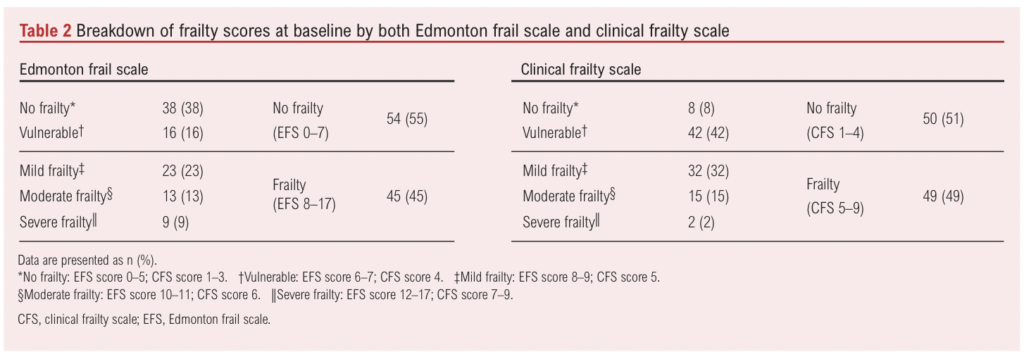
Associations of baseline patient characteristics with frailty are presented in Table 3. Frailty was associated with a higher CCI score, greater number of medications and lower haemoglobin concentration. A greater proportion of those with frailty were female (31% vs 15%), but this difference did not reach statistical significance (p=0.052). The mean ages of those with and without frailty were similar. Individuals with frailty had an almost 4-point worse SPPB score and 8.4 kg lower mean maximum grip strength.
Associations of frailty with trends in assessment scores over time
Twelve patients had their procedure cancelled or delayed after undergoing baseline frailty assessments and were subsequently excluded from further analysis. A higher proportion of those with frailty had their procedure delayed or cancelled, but there was no significant difference in the overall management strategy (Table 3). Seventy-one patients completed the 3-month follow-up and 61 patients completed the 12-month follow-up (Figure 1). Six patients not followed up at 3 months were successfully followed up at 12 months, meaning 55 patients completed all additional frailty and cognitive assessments per protocol.
Thirty-one patients received face-to-face clinic follow-up at 3 months. Frailty at baseline was not associated with change in median MoCA score (+1 (IQR –3 – +3) vs 0 (IQR –2 – +1); p=0.856) nor change in mean maximum grip strength (+0.1 (SD 3.1) kg vs –0.6 (SD 4.8) kg; p=0.870). Those with frailty at baseline had a greater improvement in mean (SD) SPPB score (+1.6 (3.6)) than those without frailty (–0.6 (2.5)), although this difference did not reach statistical significance (p=0.087). Caution is required in interpreting these results as fewer patients with frailty attended face-to-face clinic follow-up at 3 months (8 frail (21%) vs 23 non-frail (47%)).
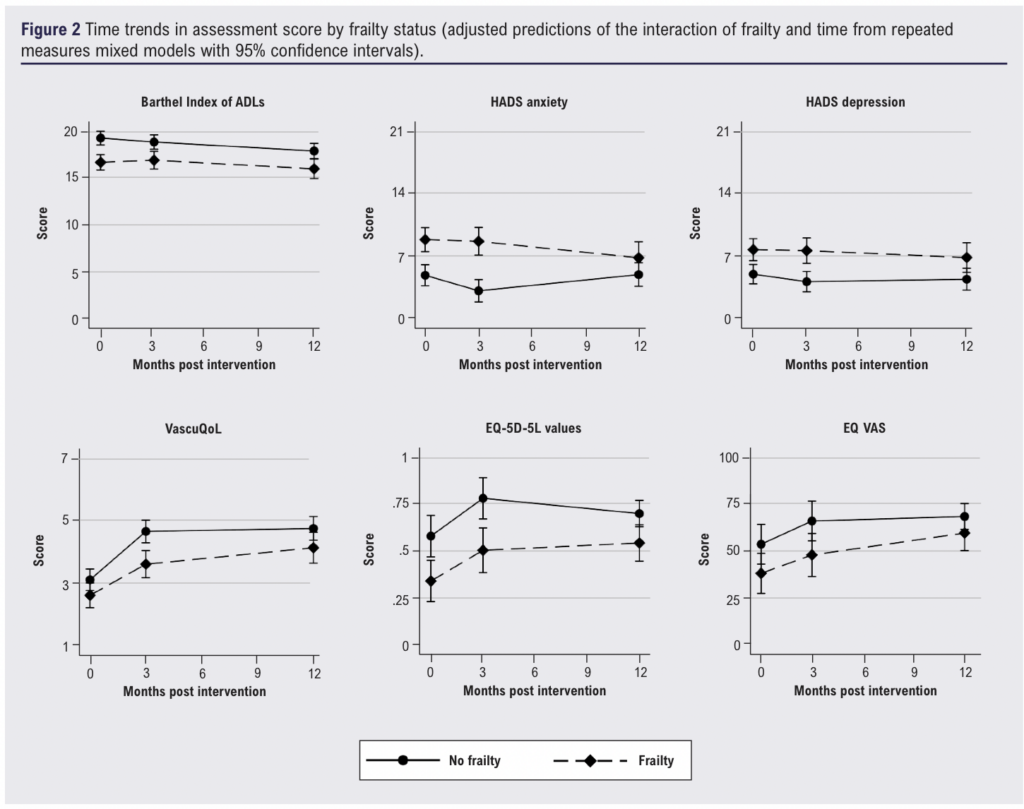
Frailty was associated with consistently worse disability, mood, QoL and cognitive function assessment scores at baseline, 3-month and 12-month follow-up (Figure 2 and Table 4). Barthel index scores worsened slightly over 12 months for both frailty and non-frailty groups (p<0.001). Individuals without frailty had a greater improvement in both VascuQoL and EQ-5D-5L scores at 3 months but little further change at 12 months, whilst those with frailty saw further improvement in QoL scores at 12 months. The time trends for the improvement in VascuQoL scores (p<0.001), EQ-5D-5L values (p=0.001) and EQ visual analogue scale (p=0.001) were statistically significant, but there was no difference in time trends by frailty status. Individuals without frailty had an improvement in HADS anxiety scores at 3 months that was lost by 12 months, whilst those with frailty demonstrated little change at 3 months but an improvement at 12 months. This difference in trends in HADS anxiety scores over 12 months was the only statistically significant interaction of frailty status and time (p=0.014). Sensitivity analyses showed no difference in results.
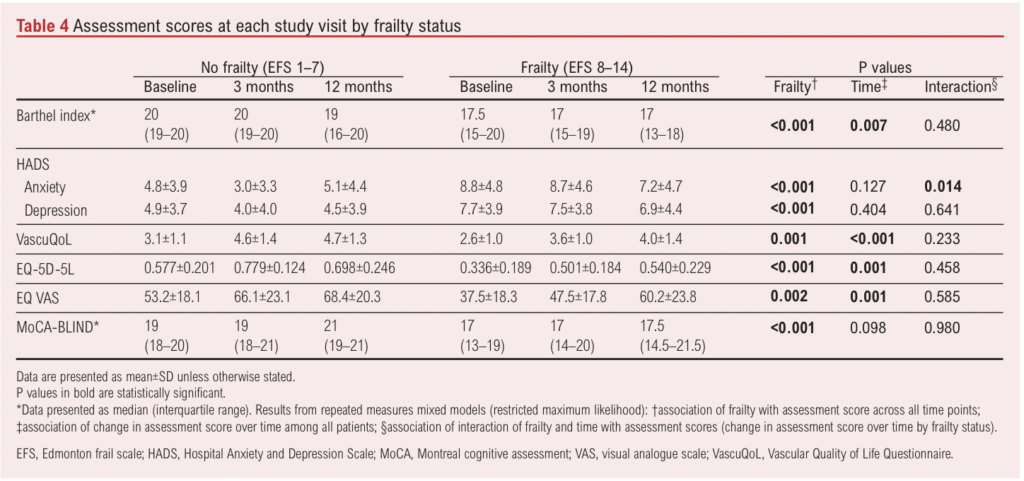
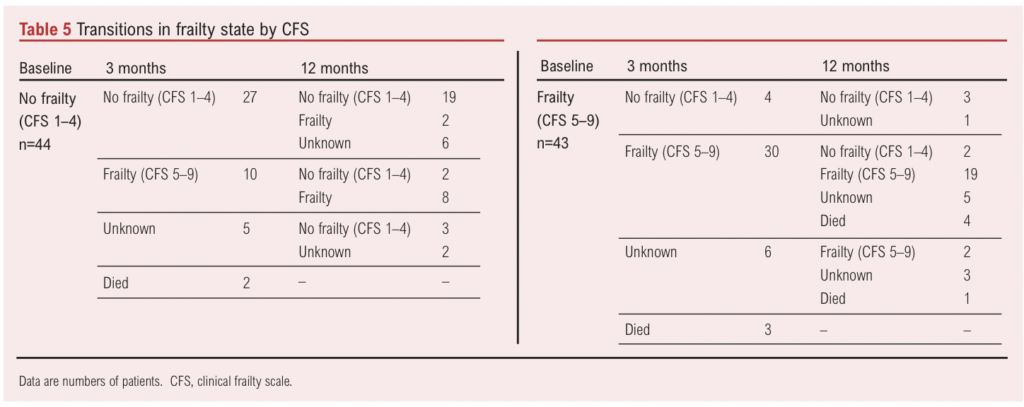
Investigation of frailty trajectory was only possible using the CFS, as the EFS cannot be completed via telephone. Forty-four patients were classified as non-frail (CFS <4) at baseline. Of these, two had died and 10 (23%) had transitioned to frail (CFS >5) at 12 months (missing data for eight patients) (Table 5). Conversely, of 43 patients classified as frail (CFS >5) at baseline, eight had died and five (12%) had transitioned to non-frailty (CFS <4) at 12 months (missing data for nine patients). Mean (SD) age at baseline was older in those classified as frail by the CFS at 12 months (74.7 (10.0) years vs 67.2 (9.0) years; p=0.003). Those transitioning from non-frailty to frailty were older than those who remained non-frail at 12 months (mean (SD) age 76.7 (8.1) years vs 67.8 (9.4) years) whilst those transitioning from frailty to non-frailty were younger than those who remained frail at 12 months (mean (SD) age 64.3 (7.2) years vs 73.7 (10.9) years).
Associations of frailty with survival and major amputation at one-year
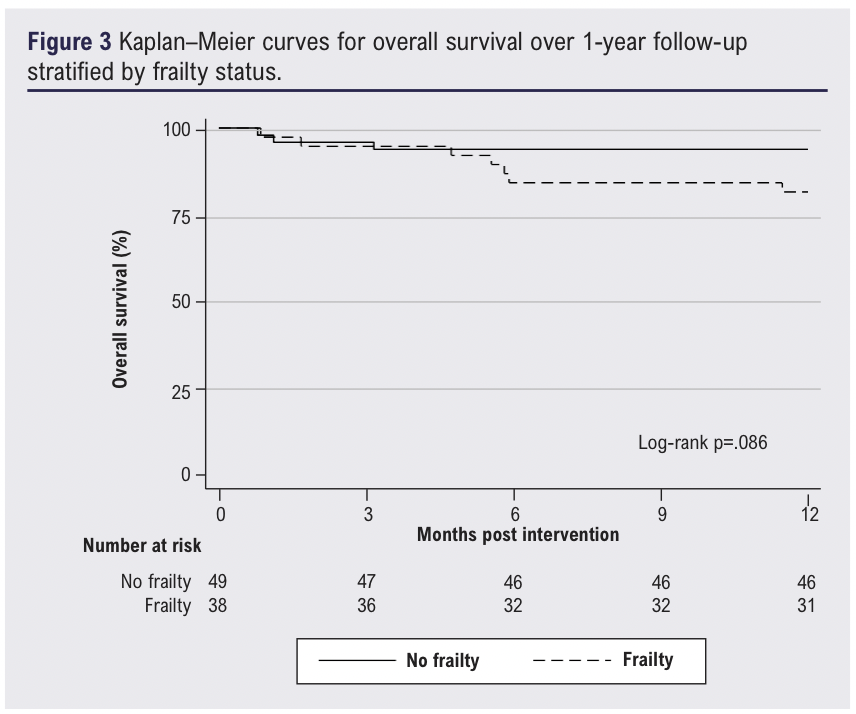
At 12 months, 10 patients (11%; 7 frail, 3 non-frail) had died and 11 patients (13%; 5 frail, 6 non-frail) had undergone a major amputation. There was a trend towards worse survival among those with frailty (Figure 3), but this was not statistically significant (p=0.086). Increasing age (HR 1.13, 95% CI 1.04 to 1.24; p=0.007) was independently associated with 12-month mortality on multivariable analysis, whilst frailty was not (HR 4.04, 95% CI 0.80 to 20.40; p=0.090). There was no association of frailty with major amputation at 12 months (SHR 0.62, 95% CI 0.16 to 2.46; p=0.498) on multivariable analysis.
Discussion
This study represents a detailed investigation of frailty in patients with CLTI. Around half had frailty at baseline, consistent with previous research.27 Frailty was strongly associated with comorbidity, polypharmacy, cognitive impairment, disability and lower limb dysfunction, as well as worse mood and QoL. This is anticipated given their contribution to both the phenotype and cumulative deficit models of frailty, and the EFS includes direct assessment of these domains.1,2,8,26 The results demonstrate the interrelatedness of frailty with both disability and comorbidity in the context of CLTI, as reported in other populations of older adults.8 The EFS (which includes timed up-and-go test) had reasonable agreement with the CFS in identifying frailty but classified a higher proportion of patients as ‘no frailty’ than ‘vulnerable’ (Table 2). Those with frailty (by EFS) also had significantly lower grip strength. These findings suggest that the EFS score is not unduly influenced by CLTI-related lower limb dysfunction and support its validity as a frailty assessment in those with CLTI. This is an important finding given concerns regarding overestimation of frailty in CLTI patients by measures (such as the EFS) that include assessment of mobility.28
Importantly, this study is novel in describing the interactions of frailty status, disability and QoL over time following intervention for CLTI. There were modest improvements in QoL scores at 12 months among those with and without frailty. A recent meta-analysis also showed a small to moderate improvement in QoL over time in those with CLTI.29 Patients with frailty had significantly worse anxiety and depression scores at baseline but showed modest improvement over 12 months, whilst those without frailty showed little change. These findings demonstrate that those with frailty still have a QoL benefit from revascularisation despite worse QoL at baseline. There was also an overall trend of worsening disability scores across all patients, and nearly a third of non-frail patients transitioned to frailty at 12 months following intervention. However, data from 3-month follow-up showed improvement in lower limb function (SPPB scores) among those with frailty attending clinic follow-up. Revascularisation for CLTI improves lower limb function, with post-intervention walking ability correlating with baseline Barthel index score.10,11
A small number of patients in this study transitioned from frailty to non-frailty at 3 and 12 months following revascularisation. These patients tended to be younger. The transition of a similar proportion of CLTI patients from frailty to non-frailty following revascularisation has been previously described.12 It is possible that, in a small population of individuals with CLTI, frailty may be reversed with appropriate management. CLTI itself directly and indirectly contributes to severity of frailty due to chronic pain and inflammation, CLTI-related disability and associated social isolation.8,14,30 In a general population of older adults, frailty trajectories have been shown to slow or reverse with modest increases in exercise and nutrition.31,32 Revascularisation promotes wound healing, improved lower limb function and CLTI-related disability, as well as global improvement in function and return to normal daily activities. These may, in combination, lead to a reversal or slowing of the frailty trajectory in some individuals with CLTI. Given the small numbers of patients included in this study (and transitioning in frailty states), it was not possible to undertake a detailed analysis of factors associated with improvement or decline in the frailty state. However, results from the 2-year follow-up of the LIMb study primary cohort will provide further evidence of frailty trajectories among those with CLTI.13
Collectively, these results show an improvement in QoL scores post intervention in both those with and without frailty despite an overall slight worsening in disability scores and a trend to increasing prevalence of frailty at 1 year. Decision making in CLTI is complex with guidelines recommending assessment of patient risk, severity of disease and anatomy of arterial disease.14 Current evidence of the risk–benefit balance of intervention in CLTI predominantly focuses on risk of death or major amputation.33,34 However, older people, particularly those with frailty, often value QoL and functional independence over mortality.35 It is encouraging that, overall, both those with and without frailty showed a benefit in QoL from intervention in this cohort. However, further research is needed to delineate prognostic factors associated with better and worse functional outcomes among vulnerable CLTI patients to better inform shared decision making.
Strengths and limitations
The strengths of this study are its prospective nature and comprehensive assessments of frailty and related domains. There are several limitations. This was a single-centre study, limiting the generalisability of the results and conclusions. Despite best efforts, some patients had missing follow-up data at 3 and/or 12 months, and missingness of data is assumed to be random in the mixed models. However, no difference in results was demonstrated on complete case analysis. Whilst recruitment to the LIMb study was good, 27% of eligible patients declined to participate and 28% of those recruited to the LIMb study who were eligible for the frailty and cognitive additional assessments declined to participate (Figure 1). The overall prevalence of cognitive impairment (33%) was lower than anticipated.9 Additionally, age, surprisingly, was not associated with frailty at baseline by either the EFS or CFS and few patients included in this study had severe frailty (Table 2). This may be due to selection bias, as those managed conservatively (ineligible) and those who declined to participate may have had a greater degree of frailty and/or cognitive impairment. Given the numbers of patients and events, it was necessary to dichotomise frailty scores to compare those with and without frailty. However, frailty is a continuum with greater degree of frailty conferring greater risk of poor outcome and should ideally be analysed as an ordinal variable. As few included patients had severe frailty, dichotomisation is unlikely to have significantly impacted the result and conclusions. The main limitation was the impact of COVID-19. Necessary recruitment pauses resulted in two-thirds of the target 150 patients recruited. Follow-up restrictions precluded face-to-face follow-up for most patients and EFS cannot be completed via telephone. CFS is based on history (self-report) alone and has been assessed via telephone in previous research.36 Finally, the impact of COVID-19 on assessment scores during follow-up cannot be quantified. All patients underwent 12-month follow-up after the first UK wave of the pandemic and COVID-19 related restrictions (eg, lockdowns) may have worsened mood, QoL and disability independent of CLTI symptoms.
Conclusions
In CLTI patients, frailty is associated with greater disability, lower mood and worse QoL both at presentation and over 1 year post-intervention. However, both those with and without frailty showed similar improvements in QoL at 1 year. Frailty may be reversible in a small proportion of younger patients; however, people with CLTI overall will demonstrate progression in disability and frailty status over 1 year. Baseline frailty assessment in CLTI patients is important to guide prognostic discussion and shared decision making.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(3):131-139
Publication date:
May 22, 2024
Author Affiliations:
1. Department of Cardiovascular Sciences, University of Leicester, Leicester, UK
2. Leicester Vascular Institute, University Hospitals of Leicester NHS Trust, Leicester, UK
3. National Institute for Health Research Leicester Biomedical Research Centre – The Glenfield Hospital, Leicester, UK
4. Department of Population Health Sciences, University of Leicester, UK
5. MRC Unit for Lifelong Health and Ageing, University College London, UK
Corresponding author:
John Houghton
University of Leicester, Department of Cardiovascular Sciences, On-call Suite, Glenfield General Hospital, Groby Road, Leicester LE3 9QP, UK
Email: [email protected]











