ORIGINAL RESEARCH
A survey of surgical site infection prevention practice in UK vascular surgery
Lathan R,1 Yiasemidou M,2 Long J,1 Mohamed A,1 Hitchman L,1 Ravindhran B,1 Sidapra M,1 Smith GE,1 Chetter IC1
Plain English Summary
Why we undertook the work: Wound infections after surgery cause a multitude of problems for patients ranging from minor to major illness, including death in some instances. Understandably, this affects one’s ability to function day to day both physically and mentally. Further, all these unwanted problems incur additional costs to the NHS. Various measures can be taken to potentially reduce the chances of an infection after surgery, but the strength of the evidence supporting these measures is inconsistent. Previous research highlighted that the preventive measures varied from one hospital to another, and since this work, several guidelines have been produced to try to standardise these preventive measures. However, it is not known whether these guidelines have led to conformity between hospitals. We therefore aimed to survey vascular surgeons to understand their current practice for implementing measures to prevent wound infection.
What we did: We identified 15 interventions aimed at reducing wound infection after surgery from the three guidelines published from international bodies (the World Health Organisation, National Institute for Health and Care Excellence, and Centers for Disease Control and Prevention). A focus group consisting of experts in vascular surgery then constructed a questionnaire survey that would capture practice pertaining to these 15 areas. The survey underwent two rounds of validity testing in one hospital with 10 consultants in vascular surgery, before being sent to all the membership of the Vascular Society’s.
What we found: Responses were received from 109 surgeons from 47 hospitals across the UK, giving a response rate of 24%. Surgeons estimated that 7.5% of patients get wound infections after surgery. Operations involving arteries in the legs or major lower limb amputation were identified as at highest risk of infection. About half (52% of respondents) diagnose infections using their experience alone rather than recognised criteria. When cleaning the skin before an operation, more than half (57%) use chlorhexidine and 78% prefer to use an alcohol-based solution. However, the majority of surgeons (79%) prefer to use an aqueous-based solution to clean their own hands before surgery. After surgery, 74% of surgeons do not have a dedicated programme in place to monitor wounds in case of infection.
What this means: Despite three international bodies producing guidance to prevent wound infections after surgery, the practice in vascular surgery remains varied, perhaps reflecting inconsistency between guidance recommendations. Further high-quality evidence is needed to assess the effectiveness of individual interventions to reduce the incidence of wound infections after vascular surgery. The impact of this evidence should then be maximised to develop up-to-date guidance and minimise variability in practice.
Abstract
Background: Surgical site infections (SSI) have a significant impact on morbidity and mortality within vascular surgery. Despite the publication of several guidelines, there is a lack of consensus regarding the most effective perioperative practice to minimise the incidence of SSI. This study aimed to assess the current practice of SSI prevention among UK vascular surgeons.
Methods: An online survey developed using the current National Institute for Health Care and Excellence (NICE), Centers for Disease Control and Prevention (CDC) and World Health Organisation (WHO) SSI prevention guidelines was piloted in a tertiary vascular centre before being distributed by email to the members of the Vascular Society of Great Britain and Ireland. The survey contained 15 question domains across preoperative, perioperative and postoperative phases to establish current SSI prevention practice. The survey was open for 1 month with reminder emails at 2 and 3 weeks.
Results: A total of 109 respondents from 47 UK hospitals completed the survey, 90 of which were consultants (82.6%). The median reported SSI rate was 7.5% (IQR 5–10%). Lower limb arterial and major limb amputations were highlighted as the highest risk procedures of SSI. Empirical criteria are used by 67.2% of respondents to diagnose SSI, and over half (52.2%) of surgeons used this alone. Most respondents use alcoholic chlorhexidine gluconate (69.6%) skin preparation and basic wound dressings (67.6%). Around half (52.5%) of respondents reported that they would use negative pressure wound therapy for closed wounds. Formal wound surveillance was not undertaken by 73.7% of respondents.
Conclusions: There is little agreement in current guidelines on the best practice to prevent SSI. Unsurprisingly then, clinical practice follows suit and continues to show little consensus on prevention measures used. There also appears to be a disparity in registry level, clinical perception and literature data for SSI rates. Well-designed high-quality trials are needed to provide evidence-based recommendations in this field.
Introduction
Surgical site infections (SSI) are a common complication following vascular surgery, with significant detrimental effects for patients and healthcare providers.1 Reported SSI rates vary, but may be as high as 40%.2 This high rate is due to vascular surgical patients often being elderly, smokers and diabetics, frequently having multiple long-term conditions. Undesirable physical sequelae of SSI include pain, immobility, scarring, prolonged hospital stays and additional visits to clinics or from community services.3 SSI also inflict a heavy psychological burden, such as with social isolation due to odour and weeping being reported by patients as ‘embarrassing’ and leaving them in ‘utter despair’.4 Unsurprisingly, SSI are associated with depressive features, particularly with the chronicity of SSI-related illness.4,5 Vascular patients suffering from SSI incur greater length of stay, with those with infected amputation sites residing on average for 21 days compared with 3.3 days for those with infected abdominal hysterectomy.6 This concomitant increase in morbidity and prolonged hospitalisation has an adverse impact on health-related quality of life7 and survival. Furthermore, postoperative wound infections are detrimental to the surgeon-patient relationship.8 Moreover, healthcare systems sustain substantially elevated costs.9,10 Recent cost analysis in patients undergoing vascular surgery estimated the additional cost at £3,776 per SSI event.11 Prior research has demonstrated a significant variation in reported SSI rates and perioperative management of patients undergoing major lower limb amputations.12 In recent years, key organisations have published updated guidelines on SSI prevention.13-15 It is unclear whether the introduction of these SSI prevention recommendations has resulted in widespread implementation. Aiming to establish current clinical practice in the prevention of SSI within UK vascular surgery, our group designed, validated and distributed an online questionnaire regarding SSI prevention practice to the membership of the Vascular Society of Great Britain and Ireland. The insights gained from expert responses are reported in this paper.
Methods
The steering group for this study consisted of a professor, a consultant vascular surgeon, an academic clinical lecturer, a specialty trainee and a research fellow. The steering group coordinated the questionnaire development, validation, distribution and analysis.
Questionnaire development
SSI prevention guidelines from the National Institute for Health and Care Excellence (NICE), Centers for Disease Control and Prevention (CDC) and World Health Organisation (WHO) were analysed to identify 15 preventive measures forming the basis of survey question synthesis. Each guideline was also evaluated as to the level of recommendation for all SSI prevention measures to establish a rudimentary utility of the current strategies (Table 1). Survey questions for each of the 15 SSI prevention domains were discussed with the steering group, resulting in the addition of two further domains (type of sutures and post-discharge wound surveillance) and the removal of a single domain (surgical gloving) as little variation was anticipated. All decisions required unanimous agreement amongst the steering group members. Additionally, questions were reviewed to ensure wording was succinct, clear and unambiguous.
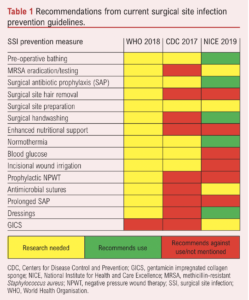
Questionnaire validation
A preliminary survey of 34 questions across 16 SSI prevention domains, including a range of binary, multiple choice and free-text open-ended questions, was validated through two rounds of piloting at a tertiary vascular centre with 10 consultants. After round 1, major (questions removed or added) and minor alterations (wording alterations) were made based on the feedback from the consultant body. Major alterations included two questions rationalised into one on three occasions, the removal of six questions and the addition of five questions. The reasons for major alterations included a lack of utility, repetition, a desire to minimise respondent fatigue, and an attempt to ensure all SSI prevention strategies were addressed. Ten minor alterations were made to wording or responses of questions. After the second pilot, no further changes were suggested.
Questionnaire distribution
The online survey was disseminated using Qualtrics XM platformTM software. Demographic data collection included background information (working grade and current hospital of practice) and the presence of a generic SSI policy within the unit (National Vascular Registry (NVR) submission, diagnostic criteria, infection rates, high-risk procedures and active SSI prevention policy). Participants were advised their responses would be anonymised. The final survey was distributed between 15 March and 12 April 2021 via email, inviting surgeon and trainee members of the Vascular Society of Great Britain and Ireland to participate. One month was allowed for responses with reminder emails circulated at 2 and 3 weeks.
Questionnaire analysis
Responses were scrutinised by the lead researcher and non-response questionnaires removed. Partially completed questionnaires were included. Only answers submitted within the response window were included in the analysis. Completed questionnaires were exported into Microsoft Excel Version 16.59. Binary responses were reported as percentages and multiple-choice answers were expressed as percentages of the specific question’s respondents. Median reported SSI estimates are presented alongside interquartile ranges. Responses including 80% or more in one item were taken as good levels of agreement for use of that practice. A χ2 test was completed where appropriate in SPSS version 27 (IBM Corp, Armonk, New York, USA) to determine the impact of variables on estimated SSI rates and a regression analysis performed. In the interest of the statistical analysis, infection rates were subdivided above and below the median reported infection rate. Estimated SSI rates collected from the survey were compared with the nationally reported rates from the NVR 2019 (with permission).
Results
Survey responses
A total of 109 respondents completed the survey, with the majority (n=90, 82.6%) identifying they were in a consultant role. Other positions included specialty registrar (n=14, 12.8%), core trainee (n=3, 2.8%) and not specified (n=2, 1.8%). Based on numbers of consultants registered with the Vascular Society (n=376), this is approximately a 24% response rate. Survey responses came from a total of 47 hospitals with a wide distribution across the UK, as shown in Figure 1. The majority of respondents (58.7%, n=62) primarily worked in a university hospital and 41.3% (n=43) in a district general hospital.
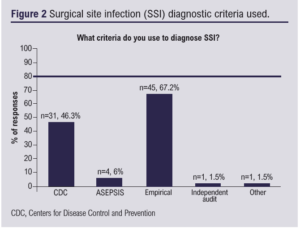
Infection rates
The median estimated SSI rate was 7.5% (IQR 5–10%). Twenty respondents (18.3%) reported SSI rates >10%. Empirical criteria alone were used to diagnose SSI by 35 (52.2%) respondents (Figure 2). Lower limb arterial and major limb amputation were identified as high-risk procedures for SSI (n=58, 92.1% and n=47, 74.6% of responses, respectively). All units routinely submitted data to the NVR and 62.7% (n=47) had a dedicated SSI prevention protocol.
Preoperative measures
Preoperative bathing
Preoperative bathing was recommended to patients by 67.1% (n=49) of clinicians, with the majority indicating standard soap as the agent of choice (54.9%, n=28). Chlorhexidine gluconate (CHG) soap, CHG cloths and other agents (Betadine, n=1; Hibiscrub, n=1; Octenisan, n=2; Stellisept, n=1) were recommended by 29.4% (n=15), 5.9% (n=3) and 9.8% (n=5), respectively. Reasons given for not advocating preoperative bathing include: not necessary (14.7%), a lack of evidence base (50.0%) and a lack of capacity to provide (23.5%). Four surgeons indicated that they used to recommend preoperative bathing but, as they now admit elective patients on the day of surgery, this is not possible.
Methicillin-resistant Staphylococcus aureus (MRSA) screening
Preoperative MRSA screening was practised by 93.3% (n=56) of vascular surgeons. Reasons for not screening patients included a lack of supportive evidence and a lack of staff or time resource capacity. For patients colonised with MRSA, the majority of responders provide treatment with both intranasal mupirocin and CHG body wash (66.1%, n=39). Intranasal mupirocin alone is used in 16.9% (n=10) and CHG body wash alone in 5.1% (n=3) of cases.
Surgical antibiotic prophylaxis (SAP)
The majority of surgeons use SAP for open abdominal aortic aneurysm (AAA) repair (95.5%, n=64), lower limb arterial disease (94.0%, n=63), major limb amputation (92.5%, n=62), endovascular aortic aneurysm repair (89.6%, n=59), carotid endarterectomy (89.6%, n=58) and minor limb amputation (77.6%, n=52) procedures. Antibiotic prophylaxis is used by less than one-third of surgeons in peritoneal dialysis catheter (26.9%, n=18), open venous (20.9%, n=13) and arteriovenous fistula (13.4%, n=7) procedures. This was a multiple response question; some respondents did not indicate use of antibiotics prior to open AAA repair, lower limb arterial disease or major limb amputation, which prevented these from reaching 100% use. SAP is given at incision by 21.3% (n=16) and within two hours preoperatively by 77.3% (n=58).
Hair removal
Only 13 responses were available for this question. Hair removal typically takes place in theatre (84.6%, n=11), with a clipper (100%, n=13). Reasons that necessitate this are to avoid hair in the closure site (84.5%), to facilitate postoperative dressings (58.6%) and for ease of skin preparation (55.2%). Only 1.7% (n=1) did not remove hair covering the incision site, although a reason was not specified.
Surgical site preparation
Most responders reported using alcoholic skin preparations (78.3%, n=54), whilst 11.6% (n=8) used aqueous and 10.1% (n=7) used both aqueous and alcoholic solutions. Chlorhexidine skin preparation was preferred by 56.5% (n=39) of respondents, povidone iodine-based solutions by 15.9% (n=11) and 27.5% (n=19) used both preparations.
Hand preparation
The majority of surgeons used an aqueous-based solution for surgical scrubbing (79.2%, n=61), 18.2% (n=14) used alcohol-based solutions and 2.6% (n=2) would use either.
Perioperative measures
Nutrition
Less than half of the respondents routinely carry out perioperative nutritional assessments (45.6%, n=26), 33.3% (n=19) do not undertake this practice and 21.1% (n=12) would do so for targeted groups (empirical decision, n=5; elective open procedures, n=2; amputation, n=1; low albumin, n=1; frailty, n=1). Reasons for not undertaking nutritional assessments included lack of time (33.3%) or staff (36.7%) to assess every patient. Furthermore, 30.0% indicated they had no nutritional service to refer to.
Temperature regulation
Methods to maintain perioperative normothermia included standard blankets (53.7%, n=36), forced air warming (86.6%, n=58), heated mattress (46.3%, n=31) and warmed fluids (50.7%, n=34).
Blood glucose
Just over half of surgeons monitor blood glucose perioperatively in patients without diabetes (57.4%, n=39).
Wound irrigation
Wound irrigation prior to closure of clean wounds was practised by 31 (49.2%) respondents, with saline (50.0%, n=22), povidone iodine (31.8%, n=14) and hydrogen peroxide (16.9%, n=7) being the most commonly used wound irrigation agents.
Gentamicin impregnated collagen sponge (GICS)
GICS was used by 71.6% (n=48) of respondents. Indications for usage included prosthetic implants (62.5%), venous implants (10.4%), biosynthetic implants (18.8%), re-intervention surgery (70.8%), contamination or known infection (12.5%) and groin wounds (2.1%).
Triclosan-coated sutures
Most respondents did not use triclosan-coated sutures (77.9%, n=53), whereas 14.7% (n=10) regularly used them and 7.4% (n=5) were unsure if antimicrobial sutures were used. Reasons for not using these sutures included a lack of evidence base (27.3%), cost (9.1%), personal choice (13.6%), being unavailable (45.5%) or an unawareness of the sutures (3.0%).
Dressings
Following primary skin closure, 96.4% (n=84) of respondents indicated they used a wound dressing. A basic wound dressing was most commonly used (52.9%, n=46), while advanced dressings (14.9%, n=13) and skin glue (20.7%, n=18) were also used.
Negative pressure wound therapy (NPWT)
Negative pressure wound therapy (NPWT) was also commonly used in specific situations following primary wound closure (52.5%, n=31). Reasons provided for the use of NPWT included re-intervention surgery (31.0%), groin surgery (51.7%), high body mass index (16.7%) and long wounds (3.4%). Reasons cited for non-use of NPWT included a lack of guidelines (34.6%), a lack of evidence base (38.5%), cost (19.2%), limited resources (3.8%) and difficulty of application (3.8%).
Postoperative measures
Prolonged surgical antibiotic prophylaxis
Prolonged surgical antibiotic prophylaxis (>24 hours) was used by 22 (37.3%) respondents, most commonly after major limb amputation (73.9%) and lower limb arterial procedures (69.6%).
Wound surveillance
Formal wound surveillance programmes were not undertaken by 73.7% (n=42) of respondents. Methods of monitoring wounds included face-to-face (85.0%), community nurse follow-up (23.3%), wound photograph at discharge (10.0%), paper questionnaire (8.3%), automated remote follow-up (5.0%), specialist vascular nurse (3.3%) and other not specified (5.0%).
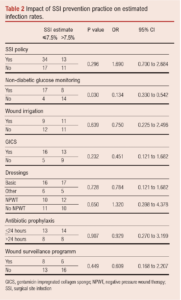
Impact of SSI prevention practice on estimated SSI rates
Participants were asked to estimate SSI rates for the period January to December 2019. The results were investigated against SSI practices for potential influencing practices, as shown in Table 2. Blood glucose monitoring in non-diabetic patients appeared to be significant in the reduction of postoperative infections.
NVR data
Data submitted to the NVR indicated a lower overall vascular SSI infection rate of 1.92% compared with the median estimated SSI rate of 7.5% (based on submissions for all AAA surgery, carotid endarterectomy, lower limb surgical revascularisation and major limb amputation).16
Discussion
This survey corroborates guideline recommendations for further well designed RCTs to evaluate the impact on SSI rates of preoperative bathing, MRSA screening, surgical site preparation solutions, perioperative blood glucose management, wound irrigation solutions, antimicrobial sutures, NPWT, gentamicin impregnated substrates, postoperative dressings and prolonged surgical antibiotic prophylaxis.13-15 This survey also highlights the disparity in reported SSI rates between registries, clinical practice and the literature. High-quality RCTs are needed to provide evidence on which to base practice and inform future versions of guidelines.
Lower limb revascularisation and amputation are both reported as high-risk procedures for SSI, which is supported by NVR data and this current survey.16,17 There is, however, little consensus on which criteria should be used to diagnose these infections, with most respondents basing diagnosis on clinical experience. Given the inconsistency in the use of diagnostic criteria, there is a need for standardisation of a robust measure to accurately diagnose SSI. CDC criteria have widely been accepted as the gold standard, but seemingly they are not uniformly used. The recently validated Bluebelle Wound Healing Questionnaire provides a promising prospect in this area, particularly given its patient reported element; however, it is not yet widely used in clinical practice.18 Current SSI prevention practice in vascular surgery varies considerably, with little consensus on many measures. Despite increasing awareness and uptake of strategies to prevent SSI, there is a clear lack of concurrence on their implementation. There is a lack of unanimity on nutritional assessment, blood glucose monitoring in non-diabetic patients, clean incisional wound irrigation, optimal wound dressings and the use of prophylactic NPWT. This seems to be recognised in the recently published CDC, NICE and WHO guidelines, which all highlight the paucity of evidence in key areas of SSI prevention.13-15 Further, not one of the 15 SSI prevention measures identified by the three regulatory bodies has a unanimously agreed recommendation for use in practice, indicating a significant need for high-quality research streams in these avenues.
There was over 80% agreement from respondents in several measures including MRSA screening, procedures requiring SAP, preoperative hair removal and use of forced air warming. However, there are aspects within those measures with little agreement in best practice, such as eradication methods for MRSA colonisation and other strategies to maintain normothermia. Although surgeons seemingly agree for which procedures to give antibiotic prophylaxis (open and endovascular AAA, lower limb arterial disease, major and minor amputation and carotid endarterectomy), only 19.4% indicated using antibiotic prophylaxis in open venous surgery. The HARVEST trial demonstrated a significant reduction in SSI with antibiotic prophylaxis in open varicose vein surgery.19 The optimal SAP regimen for vascular procedures is variable and does not appear to be evidence based. It is particularly troublesome in the lower limb arterial patient group, given the confounding issue of tissue loss and ischaemia alongside wound infection. The majority of surgeons (76%) indicated use of alcohol-based skin preparations compared with 48% reported in a 2014 survey.12 This is in line with a growing body of evidence supporting the use of alcoholic chlorhexidine over aqueous povidone iodine, although evidence from the recent FALCON trial may alter this practice.20,21 A meta-analysis of almost 12,000 participants showed that triclosan-coated sutures were clinically and cost effective in significantly reducing the risk of SSI.22,23 Despite this, our survey found that most respondents did not use these sutures for reasons of lack of evidence and expense.
The median reported surgeon SSI rate was considerably greater than the nationally reported average (7.5% vs 1.92%); however, both are significantly lower than SSI rates in the vascular literature, which can be up to 40%.2,24,26 This presents a worrying disparity between registry data, clinical practice and research findings. This survey suggests factors contributing to under-reporting may include lack of consensus regarding SSI diagnosis and lack of formal wound surveillance. Given the substantial cost identified per SSI, under-reporting misconstrues the true clinical and socioeconomic burden of this problem.11
The impact of SSI policy, non-diabetic blood glucose monitoring, wound irrigation, GICS, dressings, antibiotic prophylaxis and presence of a wound surveillance programme on estimated infection rates was investigated. The significance of blood glucose monitoring in non-diabetic patients in this survey is likely to be a type 2 error given the small sample size, although further exploration would be needed to determine the true significance of this.
Limitations
The survey achieved a response rate of almost 25% from consultant members registered with VSGBI, providing a snapshot of opinions from an extensive geographical spread of UK vascular units. It was also conducted during the COVID-19 pandemic, which may have influenced response rates. As with most surveys of this nature, it is open to responder and recall bias and results may have changed if there were more participants. In order to minimise bias, the focus group meticulously considered question wording (to ensure unambiguity) and survey length during development. A panel of experts reviewed the questions at each stage before piloting within the consultant body of a tertiary vascular unit. Response fatigue is a common issue and levels of participation did drop towards the end of the survey questions. Even with attempts to address this as made during survey development, all remaining questions were felt to be relevant to comprehensively ascertaining current practice. To preserve structure and enable ease of organisation for respondents to follow, questions were categorised into SSI-related preoperative, perioperative and postoperative themes. Additionally, not every SSI prevention measure was included in the survey – for example, surgical technique and laminar flow theatres can impact infection rates. To include all aspects of SSI prevention measures would have extended the duration of the survey and risked drop out or respondent fatigue further.
Conclusion
There is little agreement in current guidelines on the best practice to prevent SSI. Unsurprisingly then, clinical practice follows suit and continues to show little consensus on prevention measures used. There also appears to be a disparity in registry level, clinical perception and literature data for SSI rates. Well-designed high-quality trials are needed to provide evidence-based recommendations in this field.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2022;1(4):117-123
Publication date:
June 29, 2022
Author Affiliations:
1. Academic Vascular Surgical Unit, Hull University Teaching Hospitals NHS Trust, Hull, UK
2. Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK
Corresponding author:
Dr Ross Lathan
Academic Vascular Surgical Unit Vascular Labs, 1st Floor Tower Block, Hull Royal Infirmary,
Anlaby Road, Hull HU3 2JZ, UK
Email: [email protected]











