CASE REPORT
First reported case of successfully deploying the GORE Thoracic Branch Endoprosthesis under local anaesthesia
Hennessy M,1 Hussey K,2 Robertson E3
Abstract
Thoracic endovascular aneurysm repair (TEVAR) is an effective treatment option for thoracic aortic penetrating atherosclerotic ulcers (PAU) in both elective and emergency situations. The benefits of TEVAR over open surgery include reduced morbidity, length of hospital stay and short-term mortality. The GORE Thoracic Branch Endoprosthesis (TBE) increases the breadth of pathology that can be treated entirely percutaneously by allowing zone 2 TEVAR with preservation of the left subclavian artery. As it is an off-the-shelf device, it can be used in urgent cases. We present the first TBE deployed for PAU under local anaesthesia in the UK.
Introduction
Guidelines recommend thoracic endovascular aneurysm repair (TEVAR) for complicated (refractory/recurrent pain) and large penetrating atherosclerotic ulcers (PAU)1,2 which may indicate a high risk of rupture.3,4 TEVAR is an established treatment for PAU. When a zone 2 TEVAR is performed, observational data suggest that left subclavian artery (SCA) revascularisation does not reduce neurological complications.5 However, when combined with long aortic coverage (>30 cm), left SCA revascularisation should be considered in an attempt to reduce the risk of spinal cord ischaemia.6 Custom-made devices are available but, due to manufacturing times, are unsuitable for urgent cases.7 The GORE Thoracic Branch Endoprosthesis (TBE) (WL Gore & Associates Inc, Flagstaff, Arizona, USA) is a commercially available off-the-shelf device with a single retrograde side branch. Its Instructions For Use (IFU) currently define its indication being in zone 2 of the aorta. The first European implantation of the device occurred in early 2024 and the device launched in the UK later that year.8
Case report
A 58-year-old woman presented with chest pain radiating to the back 6 weeks after emergency ascending aortic and aortic valve replacement and a single coronary artery bypass graft for type A acute aortic syndrome with ascending aortic intramural haematoma and haemopericardium. Medical history included atrial fibrillation since the cardiothoracic surgery, chronic obstructive pulmonary disease, hypertension and Hartmann’s procedure for perforated sigmoid diverticulitis 6 months previously. She smoked 10 cigarettes per day. The patient was morbidly obese (BMI 38), apyrexial and deconditioned since cardiothoracic surgery. All blood indices were normal, FEV1/FVC ratio was 65% and left ventricular ejection fraction was 50–55%. Computed tomography (CT) demonstrated a new penetrating atherosclerotic ulcer (PAU) on the underside of the aortic arch. After CT and following discussion with the patient, she was transferred to our vascular unit.
The PAU was a new finding (not present on the original CT scan 15 weeks earlier) with a depth of 10 mm and width of 21 mm. It was an isolated lesion, located 15 mm beyond the origin of the left SCA. There were no clinical or laboratory signs of infection to suggest it may be mycotic. The two CT scans are shown in Figure 1. The aorta was maximally 29 mm between the left SCA and the left common carotid artery (CCA). Beyond the left SCA there was no aortic measurement above 26 mm. There was 16 mm of length between the middle of the left SCA and the proximal left CCA. The calibre of the left SCA was 10 mm. These measurements were suitable for a 31 mm TBE device with an 8 mm portal.
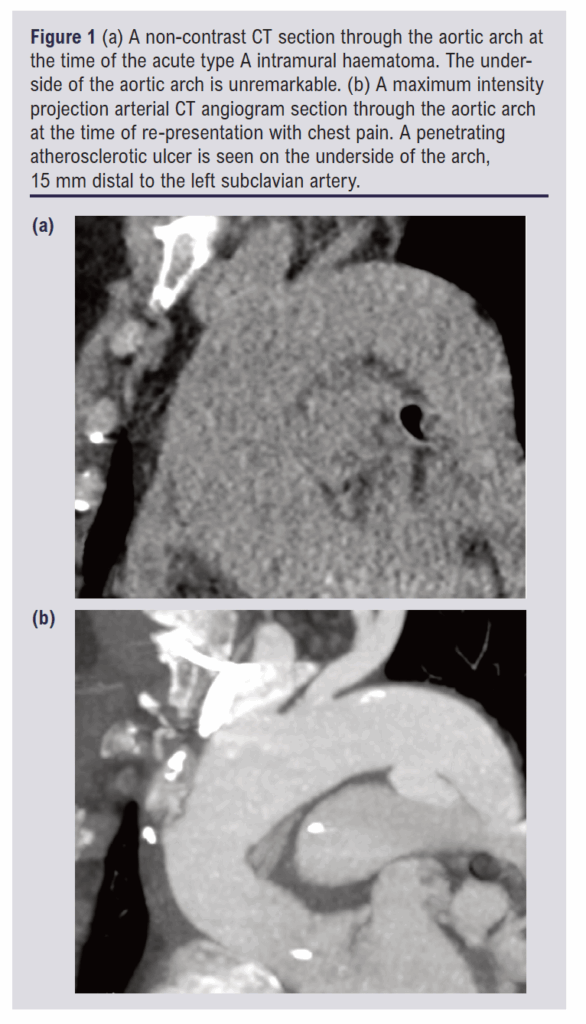
A labetolol infusion was commenced for blood pressure control and nicardipine was added over the first night. The patient was brought to theatre and positioned with the left arm out for access. Flucloxacillin (2 g) was given as antibiotic prophylaxis. An equal mixture of 0.5% levobupivocaine and 1% lidocaine hydrochloride was used for local anaesthesia, 20 mL around the femoral artery and 3 mL around the brachial artery. This was augmented with a remifentanyl infusion commenced at a targeted plasma concentration of 0.7 ng/mL and increased to 1.4 ng/mL during the procedure. 3,000 International Units of heparin were given intravenously when the large femoral sheaeth was in place. The activated clotting time was not monitored.
The left common femoral artery was punctured under ultrasound guidance and we proceeded to perform ‘pre-close’ in our standard fashion. A 180 cm Bentson wire (Cook Medical, Bloomington, Indiana, USA) was inserted and the arteriotomy was then dilated with the 7 Fr dilator of a 7 Fr Radifocus Introducer II (Terumo, Tokyo, Japan). Two Perclose ProStyle Suture-Mediated Closure and Repair Systems (Abbott Cardiovascular, Plymouth, Minnesota, USA) were deployed in turn and the 7 Fr sheath was then inserted. A Vanschie 1 Beacon Tip Angiographic Catheter (Cook Medical) was passed over the wire and that wire was then exchanged for a 300 cm Lunderquist Extra-Stiff Double-Curved Wire Guide (Cook Medical) which was advanced to the aortic valve. The 7 Fr sheath was then removed and replaced with a 20 Fr 33 cm DRYSEAL Flex Introducer Sheath (WL Gore & Associates Inc) which was hubbed at the skin.
During the pre-close steps, a second operator punctured the left brachial artery under ultrasound guidance. A 5 Fr Radifocus Introducer II was inserted over the Bentson wire until it was in the aortic arch. Our standard technique to access the descending aorta from the left subclavian is to pass a 5 Fr TEMPO Pigtail catheter (Cordis, Miami Lakes, Florida, USA) over the Bentson wire until the pigtail is in the aortic arch. The pigtail can then be torqued to face the descending aorta. When the Bentson is re-advanced through the pigtail, it begins to uncurl towards the descending aorta as the 7 cm floppy tip of the wire passes around it. The tip of the Bentson forms a large ‘J’-shaped leading edge and, when the stiff portion of the wire begins to push through the pigtail, the wire and catheter often prolapse down the descending aorta. When sufficient wire has progressed down the aorta, the catheter can be tracked over it. This series of steps occurred with ease in this case. Next we passed a 260 cm Radifocus Guide Wire M Standard Type (Terumo, Tokyo, Japan) down the descending aorta to the level of the top of the DRYSEAL sheath. Parallel to the Lunderquist wire, a 6 Fr 30 mm ONE Snare Endovascular Snare System (Merit Medical Systems, South Jordan, Utah, USA) was passed through the adjustable valve of the DRYSEAL sheath and the Terumo wire was quickly snared, giving through-and-through access between the left femoral and left brachial arteries.
Angled angiography was performed to delineate the left SCA. A 31 mm calibre TBE with an 8 mm internal portal was inserted over the Lunderquist and Terumo wires and advanced to line up the portal markers with the SCA. There was mild wire wrap which was relieved using the simple measure of retracting the device to the descending aorta, turning it and re-advancing it. This was only required once. With the TBE in place, it was deployed by one operator while the second operator fixed the delivery system at the hub of the DRYSEAL sheath. A 12 mm calibre, 60 mm length side branch component was then inserted from the femoral side of the Terumo wire while the Vanschie catheter was inserted from the brachial side of the Terumo wire. With these two devices tip-to-tip, the side branch component was advanced into the portal and then the SCA and deployed. A 10 x 20 mm Advance 35LP Low-Profile PTA Balloon Dilatation Catheter (Cook Medical) was advanced from the femoral access. The portal overlap was ballooned first, then the distal end of the side branch component, then the middle, as per the manufacturer’s recommendations. No ballooning of the TBE was performed. Angiography was repeated, confirming exclusion of the PAU and patency of the left SCA branch. Pre- and post-deployment angiograms are shown in Figure 2. The anaesthetic chart records the procedure as commencing at 11:55 and finishing at 12:50.
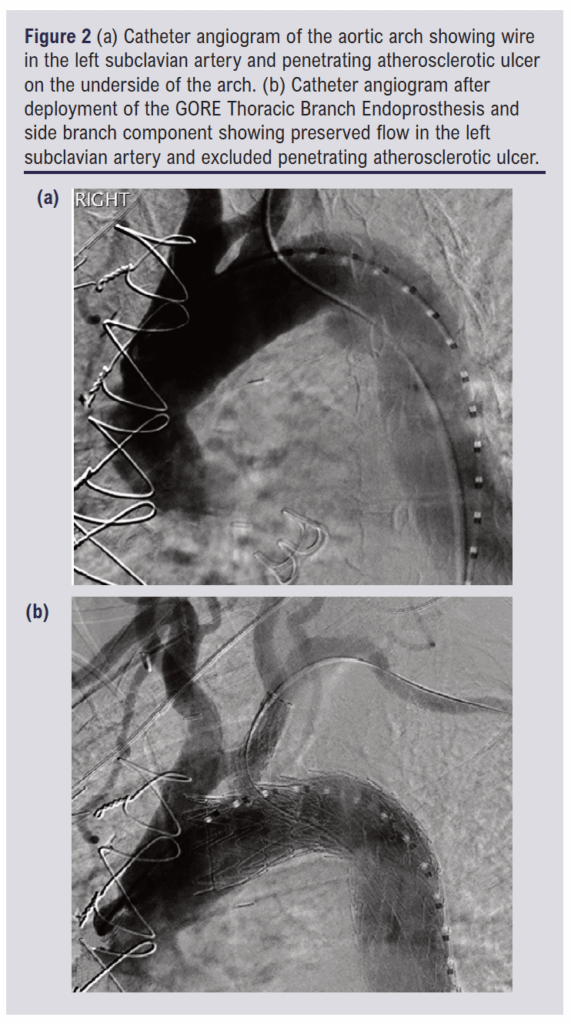
The day after the procedure the patient experienced new back pain and a CT scan was performed. Back pain after TEVAR is common and there was no cause for concern on the CT. It confirmed the exclusion of the PAU with no endoleak and good side branch flow. An image from the CT scan is shown in Figure 3. Both the pain that caused the admission and the post-procedure back pain settled quickly. The anti-hypertensive medication was switched to oral one day after the procedure. The patient was discharged home four days after the TBE.
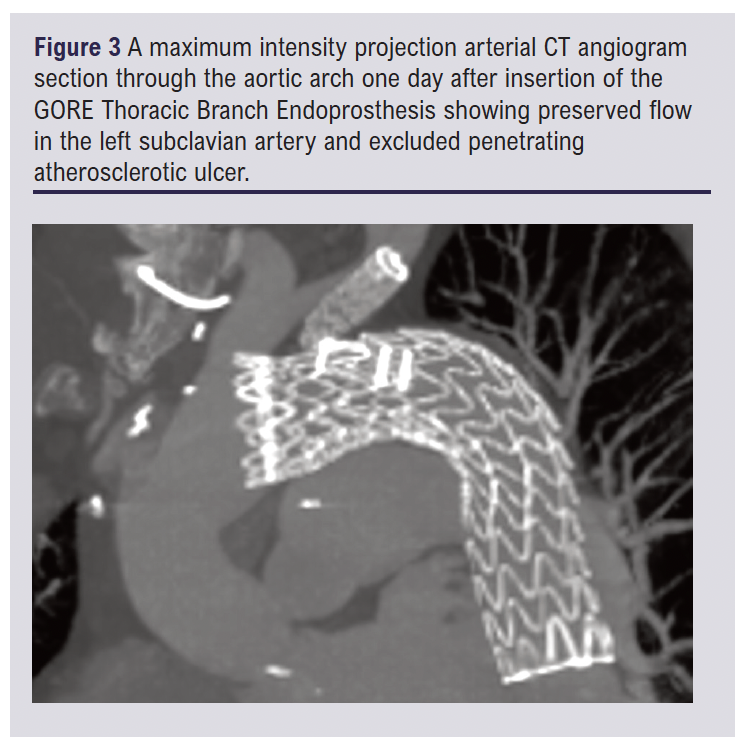
Discussion
For a symptomatic co-morbid patient with a complicated PAU, TEVAR is the preferred treatment option. TEVAR is achievable under local anaesthesia and is often standard practice.9 To achieve adequate seal as per the manufacturer’s IFU, the left SCA would have been covered. In a deconditioned co-morbid patient, local anaesthesia is preferable to general anaesthesia and, in the context of TEVAR, allows early assessment for spinal cord ischaemia.1 The TBE device allows endovascular preservation of the left SCA, but it is a more complex procedure than TEVAR and we demonstrate it is feasible under local anaesthesia.
Two key areas for success in local anaesthetic aortic cases are procedural efficiency and the correct choice of medication. Procedural efficiency includes ensuring the ready prepared availability of all devices, catheters, sheaths, etc. Multiple experienced operators helps in this regard. Regarding medication, remifentanyl infusion, occasionally augmented with small doses of benzodiazepines, appears to be superior to a low-dose propofol infusion in maintaining a predictable degree of sedation and avoiding patient agitation. Using the Minto model for achieving stable plasma concentrations of remifentanyl, we have found we can perform most endovascular aneurysm repairs (EVAR), TEVAR and complex EVAR with similar doses to those used in this procedure, and little other medication.9-11
The manufacturer’s IFU suggests a 22 Fr sheath should be required for a TBE of 31 mm calibre. Since the device has become widely used, it has quickly become apparent that a single down-sizing of sheath size is achievable with most TBE devices, as we did here. It must be noted that, while a TBE can usually be advanced into a sheath one size smaller than stated in the IFU, it cannot be retracted back out of that size sheath. Once the device is inserted, you are committed to deploying it. Another tip described in the literature is that a 5 Fr catheter or a guide sheath can be advanced from the brachial or radial access to effectively ‘dock’ with the side branch component, which helps it track through the portal into the left SCA.12
Compared to the TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System (cTAG) (WL Gore & Associates Inc), the TBE lacks some desirable features. The asymmetrical nose cone of the cTAG which orientates the outer aspect of the cTAG to the outer curve of the aortic arch is missing. However, the through-and-through left SCA wire usually assists with this orientation. There is no active angulation control to minimise ‘bird-beaking’ on the inner curve. The initial deployment of the TBE is a complete deployment, as opposed to cTAG which initially opens to 50% of its full calibre. Despite these differences, TBE remains relatively easy to use and reliable to deploy.
Both the USA and the European IFUs for TBE state that it is indicated for use in zone 2 of the aorta. However, it is licensed for use in zones 0 and 1 in the USA and reports of its use in these zones are encouraging.13,14
Conclusion
TBE offers an off-the-shelf solution to a broader range of acute aortic syndromes than standard TEVAR and it preserves the left SCA. Despite the added complexity of a side branch, the procedure can still be performed successfully under local anaesthesia.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2025;4(4):234-237
Publication date:
August 28, 2025
Author Affiliations:
1. Department of Interventional Radiology, NHS Greater Glasgow & Clyde, Queen Elizabeth University Hospital, Glasgow, UK
2. Department of Vascular Surgery, NHS Greater Glasgow & Clyde, Queen Elizabeth University Hospital, Glasgow, UK
3. Department of Anaesthetics, NHS Greater Glasgow & Clyde, Queen Elizabeth University Hospital, Glasgow, UK
Corresponding author:
Dr Martin Hennessy
Department of Interventional Radiology, NHS Greater Glasgow & Clyde, Queen Elizabeth
University Hospital, 1345 Govan Road, Glasgow, G51 4TF, UK
Email: Martin.Hennessy@ nhs.scot











