ORIGINAL RESEARCH
Management of VTE following superficial endovenous treatment: a global survey
Whittley S,1 Onida S,1,2 Carradice D,3,4 Davies AH1,2
Plain English Summary
Why we undertook the work: Varicose veins can be treated with procedures inside the veins near the skin, which can sometimes lead to blood clots in the deeper veins (deep vein thrombosis, DVT). To reduce the risk of DVT, some patients are given blood-thinning medications. However, there is not clear agreement on whether these medications help, what the risks of giving them are or how long to use them. We wanted to understand how healthcare professionals around the world manage the risk of DVT and how they use these medications when they perform varicose vein procedures.
What we did: We created an online questionnaire for healthcare professionals who treat varicose veins. The questionnaire asked about the types of treatments they offer, when they prescribe blood-thinning medications and which ones they use. After collecting responses, we looked at the data to find patterns and see how practices vary in different countries.
What we found: We received 263 responses, mostly from vascular surgeons. The results showed wide variation in the use of blood-thinning medications when treating varicose veins. For example, healthcare professionals in North America were less likely to prescribe these medications than those in other regions. The type of treatment also made a difference – procedures that use heat were more likely to lead to the use of blood-thinning medications, while non-heat treatments were less likely to be followed by the use of these drugs, even though they might also pose a risk of DVT.
What this means: Our questionnaire suggests that there is not a standard or common way to give medications to prevent DVT (blood clots) when carrying out varicose vein treatment procedures. The use of these medications varies by region and treatment type, and there is no clear global standard. This highlights the need for more research and better guidelines to make sure that patients receive the most appropriate care.
Abstract
Introduction: Superficial endovenous intervention is the recommended treatment for symptomatic varicose veins. However, venous thromboembolism (VTE) remains a known complication. Guidelines, including those from the National Institute for Health and Care Excellence (NICE) and European Society for Vascular Surgery (ESVS), are largely opinion-based and recommend an individualised approach to pharmacological thromboprophylaxis. However, clinical practices vary: some clinicians routinely prescribe pharmacological thromboprophylaxis while others do not. This survey examined global practices in pharmacological thromboprophylaxis for superficial endovenous interventions and identified regional and treatment trends.
Methods: An online survey was distributed via professional societies and social media platforms to healthcare professionals involved in endovenous interventions. It was piloted before dissemination and included questions on participant demographics, treatment modalities and pharmacological thromboprophylaxis use. Responses were analysed descriptively, summarised as frequencies and percentages, and categorised by region and treatment type.
Results: A total of 263 valid responses were analysed, with vascular surgeons comprising the majority of responders (68%, n=178). Most participants were from Europe (64%, n=170) and North America (18%, n=48), while representation from South America (8%, n=20), Asia (6%, n=15), Africa (3% n=7) and Australasia (1%, n=3) was more limited. Ultrasound-guided foam sclerotherapy (UGFS) was the most commonly performed procedure, offered by 77% (n=141) of individuals in private, 70% (n=67) in public and 60% (n=41) in academic settings. Geographic variations were observed in pharmacological thromboprophylaxis use, with fewer North American clinicians prescribing it to average- and higher-risk patients compared to other regions. Among respondents prescribing extended direct oral anticoagulant (DOAC) thromboprophylaxis, rivaroxaban was the most common choice for both thermal (77%, n=41) and non-thermal techniques (75%, n=27), followed by apixaban (19%) and edoxaban (4% and 6%, respectively). Most respondents (69%, n=174) reported routinely risk-stratifying patients before treatment, with higher-risk individuals more likely to receive pharmacological thromboprophylaxis. The choice of DOACs for extended thromboprophylaxis showed minimal regional variation.
Conclusions: This survey highlights a global lack of consensus on VTE risk assessment and thromboprophylaxis in superficial endovenous interventions. High-quality evidence is needed to establish standardised guidelines and improve patient outcomes. The generalisability of these findings is limited, particularly in regions where no responses were collected, such as large parts of Africa, the Middle East and areas of Asia and South America. Small sample sizes in certain regions and self-reported data reliance introduce potential selection and reporting bias. These limitations highlight the need for broader, more inclusive research and robust statistical analysis to ensure globally applicable recommendations.
Introduction
Superficial endovenous interventions have become the gold standard for treating symptomatic varicose veins, with multiple guidelines recommending these procedures where appropriate.1,2 However, venous thromboembolism (VTE) – encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE) – is a recognised complication of these procedures, with reported rates of up to 3.4%.3 The severity of VTE varies widely, from asymptomatic cases with no clinical consequences to life-threatening PE or debilitating post-thrombotic syndrome (PTS).4,5 PTS, which manifests as chronic leg pain, swelling and venous skin changes, develops in up to 50% of patients with DVT.6,7
To reduce the risk of VTE following superficial endovenous interventions, several professional bodies, including the National Institute for Health and Care Excellence (NICE), the European Society for Vascular Surgery (ESVS) and the UK Royal Society of Medicine (RSM), recommend an individualised approach to pharmacological thromboprophylaxis, particularly for high-risk patients.1,8,9 In practice, approximately two-thirds of clinicians in the UK and 73% of clinicians in Ireland routinely prescribe pharmacological thromboprophylaxis for these procedures,10,11 often tailoring their approach based on patient-specific risk factors. A recent systematic review and meta-analysis of non-randomised studies suggests that pharmacological thromboprophylaxis may reduce the rate of DVT in this setting.3 However, while anticoagulation offers potential benefits, it also carries risks, including an increased risk of bleeding.12-14 Cost-effectiveness is another key consideration, particularly in resource-limited settings where the economic burden of anticoagulation must be weighed against VTE prevention.15-17 Despite its clinical significance, the evidence base for pharmacological thromboprophylaxis in superficial endovenous interventions remains limited, with few randomised controlled trials.3 Much of the current guidance is based on observational data and expert consensus, leading to uncertainty in clinical decision-making.
Despite national and international recommendations, clinical practice regarding pharmacological thromboprophylaxis in superficial endovenous interventions remains highly variable. Some clinicians administer a single dose of low-molecular-weight heparin (LMWH), while others opt for an extended course of LMWH or a direct oral anticoagulant (DOAC).11 Approximately one third of clinicians in the UK do not routinely prescribe pharmacological thromboprophylaxis.11 Several factors contribute to this variability, including healthcare infrastructure, institutional guidelines and medicolegal considerations.18-21 In particular, several high-profile media reports of deaths following varicose vein surgery have heightened concerns over litigation,22,23 leading some clinicians to adopt more aggressive prophylactic strategies, while others remain cautious due to guideline ambiguity, bleeding risks or resource constraints.24-26
This survey aims to explore global practices in pharmacological thromboprophylaxis for superficial endovenous interventions and to identify potential trends related to country of practice and treatment modality.
Methods
Survey development
An online questionnaire was developed using the Qualtrics XM platform (see Appendix 1 online at www.jvsgbi.com).27 To enhance clarity and validity, the survey underwent internal and external pilot testing with a trial manager and four vascular surgeons.28-30 Feedback from the pilot phase was used to refine the survey’s wording and structure. The final version contained 26 questions but applied logic ensured that not all participants received every question. Based on the pilot testing, the estimated completion time was approximately four minutes.
Questionnaire structure
The survey consisted of a combination of binary, multiple choice and open-ended questions. It was divided into two main sections:
1. Demographic information. This section collected data on respondents’ professional roles and countries of practice. It also examined employment sector (private, public and academic) to explore potential differences in treatment practices, given that resource availability, financial incentives and institutional policies may influence thromboprophylaxis decisions.31,32
2. Treatment modalities offered, risk stratification and thromboprophylaxis practices. This section assessed the treatment modalities offered by respondents, whether they routinely risk-stratify patients for VTE risk and their pharmacological thromboprophylaxis practices. Respondents who performed risk stratification were asked about their pharmacological thromboprophylaxis practices for patients they classified as at “average risk” and “higher risk” of VTE. The survey did not define these risk categories, allowing respondents to apply their own clinical judgment and institutional protocols. Respondents who did not routinely risk-stratify patients were asked about their thromboprophylaxis practices without differentiation by risk level.
Questions were tailored using applied logic based on previous responses to ensure relevance, minimise complexity and reduce respondent burden.30 The pharmacological thromboprophylaxis regimens presented were based on established standard practices.11,33
Survey distribution
The survey link and its objectives were disseminated through multiple channels to reach a broad audience of healthcare professionals. It was shared via emails with members of several professional societies, including the American Venous Forum (AVF), American Vein and Lymphatic Society (AVLS), European Venous Forum (EVF) and International Union of Phlebology (UIP). Additionally, the survey was promoted via social media platforms such as Twitter, and a QR code linking to the survey was displayed at the Vascular and Endovascular Issues, Techniques and Horizons (VEITH) symposium in November 2023. Survey reach was assessed through engagement metrics such as click-through rates and completion rates.
Ethics and governance
As the survey targeted healthcare professionals and did not involve patient data, formal ethical approval was not required.34 This exemption aligns with the ethical principles outlined in the Declaration of Helsinki.35 Participation was voluntary, and informed consent was implied through survey completion. To maintain respondent confidentiality, all responses were anonymised, and any potentially identifiable information was protected securely.
Questionnaire analysis
Responses were collected over a three-month period (November 2023 to January 2024) and compiled using the Qualtrics XM platform before being exported to Microsoft Excel for analysis. Only descriptive statistics were applied. Multiple-choice responses were summarised as frequencies and percentages, with regional differences and associations between treatment modalities and thromboprophylaxis use described based on respondent distribution by continent. No formal statistical analyses were performed. Open-ended responses were grouped into common themes for qualitative analysis.
Results
Survey participation
As the survey was disseminated via social media platforms, a formal response rate could not be calculated. However, the QR code was scanned 124 times, and 307 participants opened the survey, with 306 attempting to respond. After excluding incomplete submissions (n=43), a total of 263 unique and valid responses were available for analysis, yielding an approximate completion rate of 86%. This rate is considerably higher than similar online surveys in the literature,10,36 likely because the rate was calculated based on those who opened the survey rather than the total number of individuals who received the link. The number of responses varied across questions, with response counts provided accordingly.
Respondent demographics
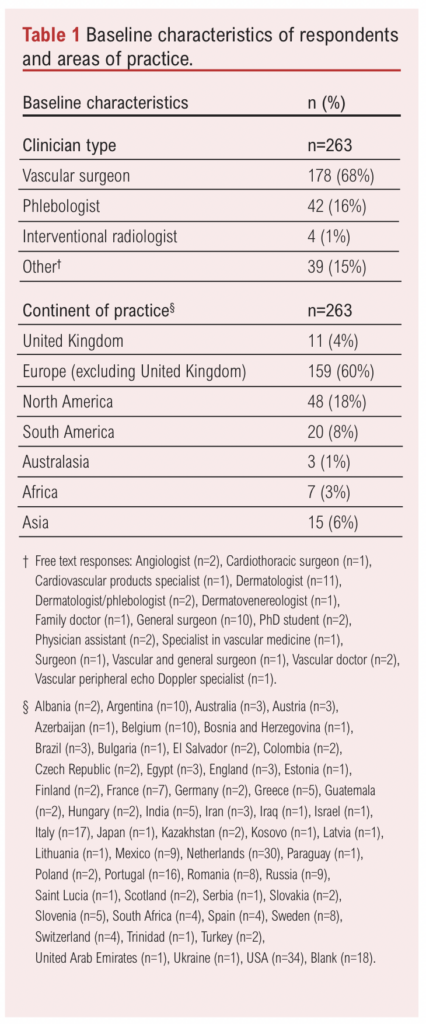
The baseline characteristics of respondents are summarised in Table 1. The majority were vascular surgeons (68%, n=178), followed by phlebologists (16%, n=42) and interventional radiologists (1%, n=4). Other professions are also detailed in Table 1. The geographic distribution of respondents is illustrated in Figure 1, with notable representation from Europe and North America but limited participation from Africa, the Middle East and areas of Asia and South America.
Practice setting and treatment modalities
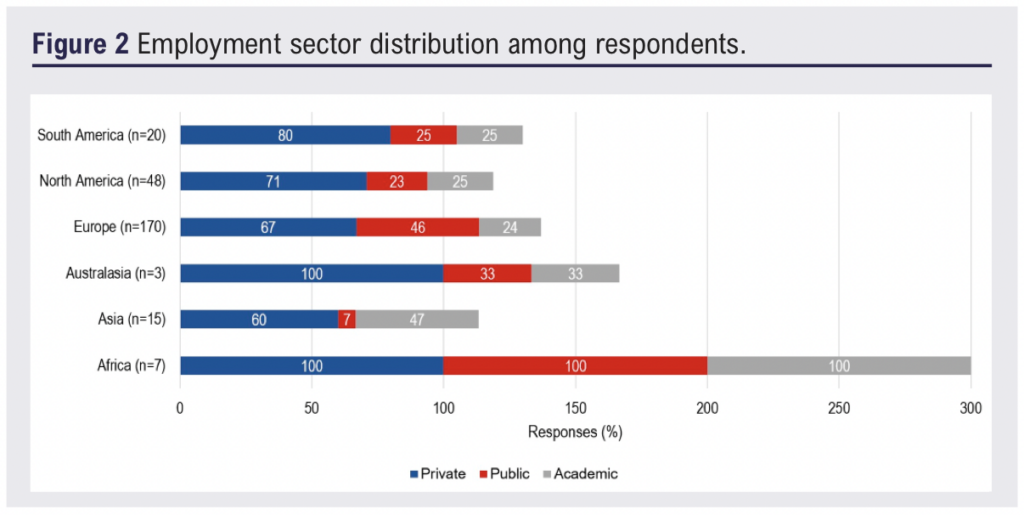
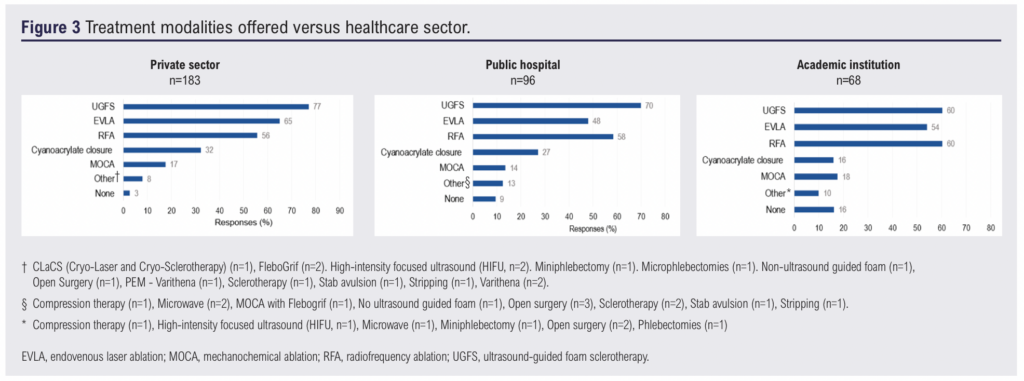
Of the 263 respondents, 70% (n=183) practised in the private sector, 37% (n=97) in public hospitals and 26% (n=68) in academic institutions. As multiple selections were allowed, many respondents reported working across more than one setting (Figure 2). Across all sectors, ultrasound-guided foam sclerotherapy (UGFS) was the most commonly offered treatment modality, reported by 77% (n=141) of private sector respondents and 70% (n=67) in the public sector (Figure 3). In academic settings, UGFS (60%, n=41) and radiofrequency ablation (RFA) (60%, n=41) were the most frequently offered modalities.
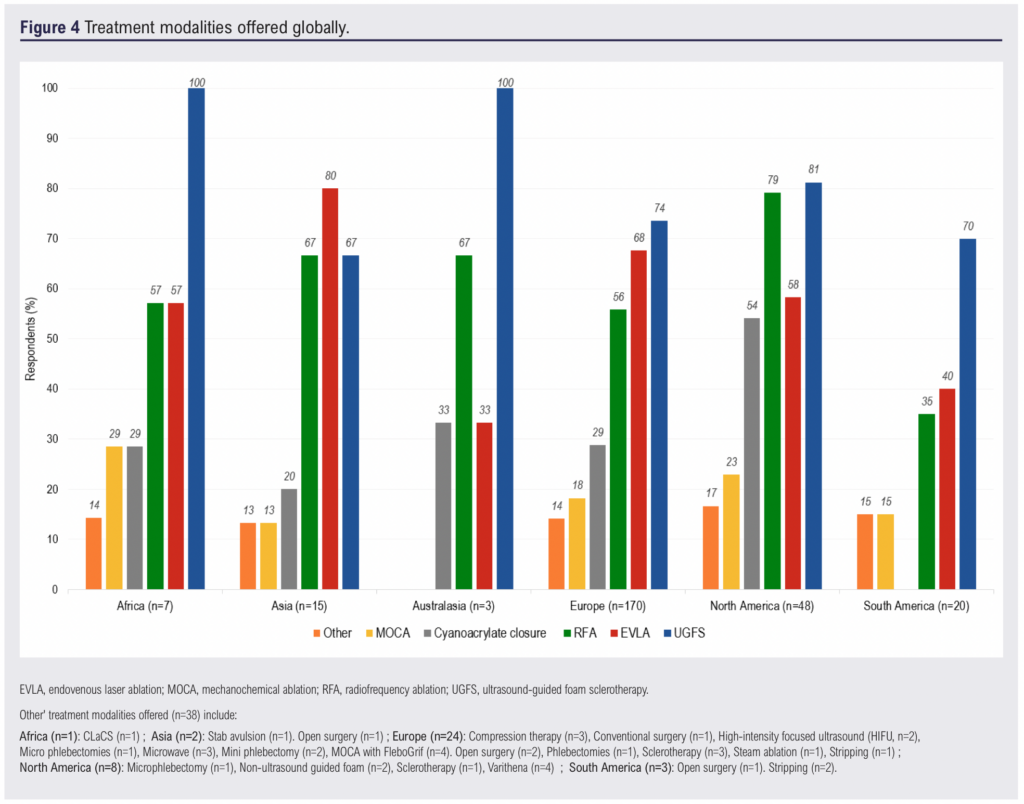
Regional variations in treatment modalities
The distribution of treatment modalities by continent is depicted in Figure 4. While UGFS was the most common intervention in most regions, endovenous laser ablation (EVLA) was predominant in Asia (80%, n=12). RFA was most widely used in North America (79%, n=38), while cyanoacrylate closure was also most common in this region (54%, n=26). Mechanochemical ablation (MOCA) and other treatments were reported less frequently across all continents.
Perceptions of compression and VTE risk assessment
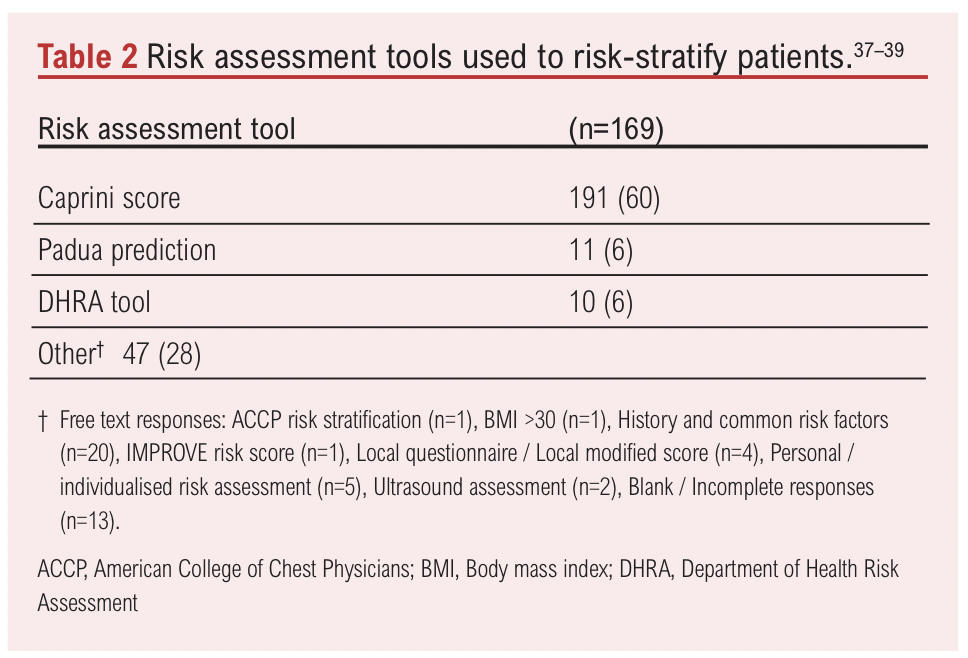
Among 257 respondents, 67% (n=172) believed that compression following endovenous varicose vein intervention serves as a prophylactic measure against VTE, while 33% (n=85) did not share this view. Regarding VTE risk assessment, 69% (n=174) of respondents reported routinely performing risk assessments before superficial endovenous interventions, while 31% (n=77) did not. The specific tools used for risk assessment are detailed in Table 2.
Thromboprophylaxis practices among risk-assessing respondents
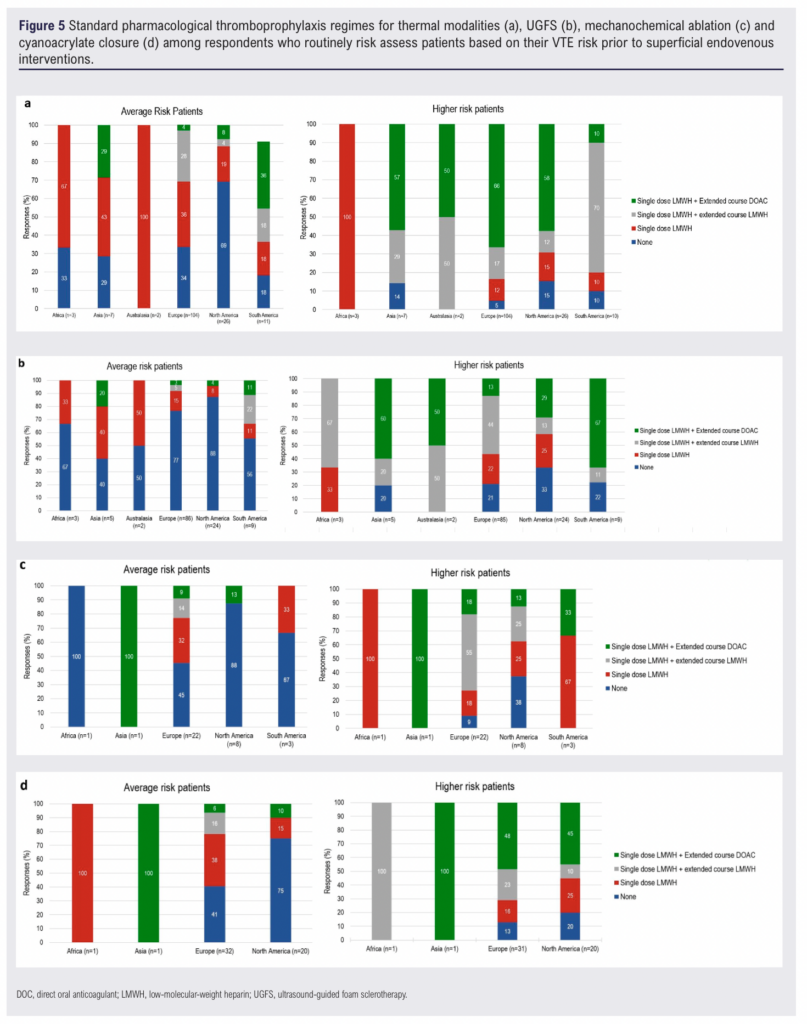
Pharmacological thromboprophylaxis regimens for different treatment modalities among respondents who routinely perform VTE risk assessments are shown in Figure 5. The data are categorised by continent and further stratified by patient risk level (‘average risk’ versus ‘higher risk’), as defined by each clinician’s own assessment. While the survey captured whether extended thromboprophylaxis was used, respondents were not asked to specify its exact duration.
Thermal ablation
In North America (n=26), 69% (n=18) of respondents did not prescribe pharmacological thromboprophylaxis for average-risk patients, but this decreased to 15% (n= 4) for higher-risk patients. In Europe (n=104), 34% (n=35) of respondents did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients, decreasing to 5% (n=5) for higher-risk patients. Similar trends were observed across Asia, South America and Africa (Figure 5a).
UGFS
For UGFS, 88% (n=21) of respondents in North America (n=24) did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients, decreasing to 33% (n=8) for higher-risk patients. In Europe (n=86), 77% (n=66) of respondents did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients, with this decreasing to 21% (n=18) for higher-risk patients (Figure 5b).
MOCA
In North America, 88% (n=7) of respondents did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients undergoing MOCA, decreasing to 38% (n=3) for higher-risk patients. In Europe (n=22), 45% (n=10) of respondents did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients, reducing to 9% (n=2) for higher-risk patients. No respondents in Asia reported prescribing pharmacological thromboprophylaxis for either average-risk or higher-risk patients undergoing MOCA (Figure 5c).
Cyanoacrylate closure
In North America, 75% (n=15) of respondents did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients, decreasing to 20% (n=4) for higher-risk patients. In Europe (n=32), 41% (n=13) of respondents did not routinely prescribe pharmacological thromboprophylaxis for average-risk patients, reducing to 13% (n=4) for higher-risk patients. No respondents in Africa reported prescribing pharmacological thromboprophylaxis for either risk category undergoing cyanoacrylate closure (Figure 5d).
Thromboprophylaxis practices among non-risk-assessing respondents
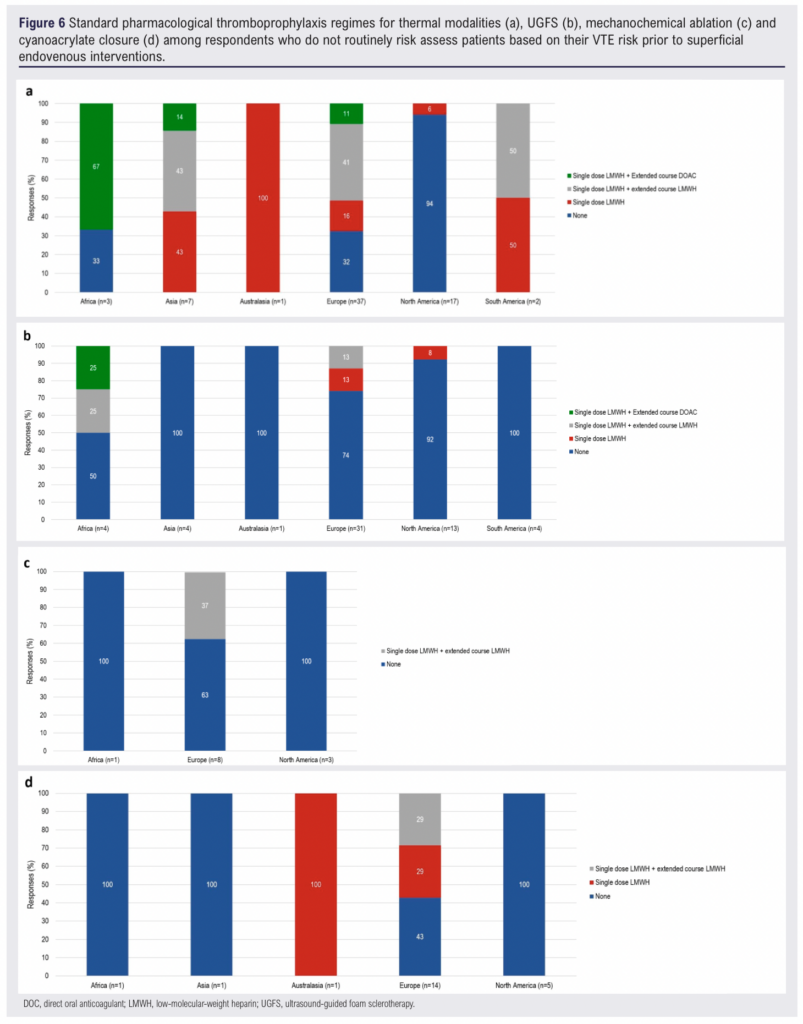
Figure 6 presents the pharmacological thromboprophylaxis regimens used by respondents who did not routinely perform VTE risk assessments prior to intervention. The data are categorised by treatment modality and continent, highlighting variations in prescribing patterns.
Thermal ablation
Among respondents who did not routinely risk assess patients, the likelihood of prescribing pharmacological thromboprophylaxis varied widely by region (Figure 6a). In North America, the majority (94%, n=16) reported not routinely prescribing pharmacological thromboprophylaxis for patients undergoing thermal ablation. In contrast, only 32% (n=12) of European respondents and 33% (n=1) of African respondents reported not routinely prescribing pharmacological thromboprophylaxis. All respondents from Asia (n=7), South America (n=2) and Australasia (n=1) reported routinely prescribing pharmacological thromboprophylaxis. However, the small sample sizes in these regions limits definitive conclusions.
UGFS
Prescribing practices for UGFS also varied considerably across regions (Figure 6b). All respondents from Asia (n=4), Australasia (n=1) and South America (n=4) reported not routinely prescribing any form of pharmacological thromboprophylaxis. Fifty percent (n=2) of African respondents indicated that they routinely prescribed extended thromboprophylaxis with either LMWH or a DOAC.
MOCA
Among the relatively small number of respondents who performed MOCA without prior risk stratification, prescribing patterns were similarly varied (Figure 6c). In both North America (n=3) and Africa (n=1), no respondents reported routinely prescribing pharmacological thromboprophylaxis. In Europe, 37% (n=3) of respondents indicated that they routinely prescribed extended thromboprophylaxis with LMWH.
Cyanoacrylate closure
The use of pharmacological thromboprophylaxis for cyanoacrylate closure was infrequent among non-risk-assessing respondents (Figure 6d). While 58% (n=8) of European respondents reported routinely prescribing pharmacological thromboprophylaxis, no respondents from North America (n=5), Africa (n=1) or Asia (n=1) reported doing so. A single respondent from Australasia indicated that they routinely prescribed a single dose of LMWH for patients undergoing cyanoacrylate closure.
Extended thromboprophylaxis regimens
Among respondents who routinely prescribed extended thromboprophylaxis with a DOAC for thermal ablation (n=53), the majority (77%, n=41) used rivaroxaban, followed by apixaban (19%, n=10) and edoxaban (4%, n=2). Similarly, among those prescribing extended DOAC thromboprophylaxis for non-thermal techniques (n=36), rivaroxaban was the most commonly prescribed (75%, n=27), followed by apixaban (19%, n=7) and edoxaban (6%, n=2).
Discussion
Regional differences
This survey provides a global perspective on current thromboprophylaxis practices, with significant contributions from clinicians in North America, Europe and parts of Asia and the Pacific. UGFS was suggested to be the predominant treatment modality across all sectors and was the predominant modality in Africa, Australasia, Europe, North America and South America. This is consistent with a 2015 survey of the Vascular Society of Great Britain and Ireland,36 which also identified UGFS as the most widely used approach for superficial endovenous treatment. While UGFS is recognised for its safety and efficacy in treating great saphenous incompetence,40 concerns remain regarding recurrence rates and lower occlusion rates compared to thermal modalities.41,42 Additionally, studies have questioned its cost-effectiveness within publicly funded healthcare systems such as the UK National Health Service,43 highlighting the need for further research into its long-term clinical and economic outcomes. UGFS is also associated with complications such as hyperpigmentation, telangiectatic matting and, in rare cases, cutaneous necrosis.44,45 Notably, larger foam sclerosant volumes have been linked to an increased risk of thrombosis, raising questions about the balance of efficacy and safety in procedures.46-50
Economic factors also play a role in thromboprophylaxis practices. The cost differences between DOACs and LMWH, along with variations in healthcare funding structures, likely influence prescribing decisions across different regions.21,51–53 In countries with limited access to DOACs or where out-of-pocket costs are high, clinicians may be more conservative in their use of pharmacological thromboprophylaxis.54,55
Compression therapy
Although the primary focus of this survey was pharmacological thromboprophylaxis, the inclusion of the compression therapy question provides additional insight into clinicians’ broader approaches to VTE prevention. The role of postoperative compression in reducing VTE risk remains an area of debate.8,56-58 In this survey, two thirds of respondents believed that compression lowers VTE risk, suggesting that mechanical measures may influence decisions regarding pharmacological thromboprophylaxis. Current NICE guidelines recommend mechanical prophylaxis for patients who are undergoing varicose vein surgery, who are at an increased risk of VTE and in whom pharmacological thromboprophylaxis is contraindicated.8 However, this recommendation is based on low-quality evidence, including studies with considerable risk of bias, imprecision and methodological limitations.59-61 Despite this, postoperative compression remains widely used in the UK and other regions following superficial venous interventions,11,36 not only for VTE prevention but also to reduce bruising, haematoma formation and pain and to improve treatment success.11,62–65 The 2023 SVS, AVF and AVLS joint guideline recommends at least one week of post-procedural compression following thermal ablation for pain reduction,58 but does not specifically endorse its use for VTE prophylaxis. The discrepancy between clinical belief and evidence regarding the role of compression in VTE prevention highlights the need for further research. While this survey suggests widespread confidence in its benefits, one third of respondents expressed doubts about the efficacy of compression in VTE prophylaxis. This aligns with prior studies reporting near universal adoption of compression,10,11 yet an ongoing debate remains about its necessity.
Pharmacological thromboprophylaxis and risk stratification
Pharmacological thromboprophylaxis in superficial endovenous procedures also remains controversial. While guidelines typically advocate an individualised approach to VTE prevention,1,8,9,58 the evidence supporting this approach in this specific context is limited. Additionally, the absence of a validated risk assessment tool for this specific patient population presents significant challenges. This survey highlights substantial variability in how clinicians define ‘average risk’ and ‘higher risk’ patients, suggesting that individual interpretation plays a major role in decision-making.
Existing tools, such as the Caprini score,39 the Department of Health Risk Assessment tool and the Padua prediction model,37,38 address general thrombotic risk but may not fully account for procedure-specific factors.10,33 Furthermore, some studies suggest that risk-adjusted anticoagulation may not alter thrombotic outcomes significantly in this setting.66,67 High-quality evidence is essential to determine the true benefits of individualised thromboprophylaxis and to develop a dedicated risk assessment tool tailored to superficial endovenous procedures. The ongoing THRIVE trial (THRomboprophylaxis in Individuals undergoing superficial endoVENous intervention),68 a large randomised controlled trial, aims to address this evidence gap. By providing robust Grade A evidence, this trial has the potential to refine thromboprophylaxis practices and offer much needed clarity in this debated area.
Regional variability in pharmacological thromboprophylaxis
This survey highlighted notable regional differences in prescribing practices for pharmacological thromboprophylaxis. Compared to other regions, North American clinicians were less likely to prescribe pharmacological thromboprophylaxis for both average-risk and higher-risk patients. This aligns with the joint guideline from the American SVS, AVF and AVLS,58 which assigns a Grade 2C recommendation for anticoagulation only in high-risk patients undergoing endovenous ablation. The Grace 2C classification reflects low-quality evidence and significant heterogeneity in existing studies. While the guideline acknowledges potential benefits of pharmacological thromboprophylaxis, it also emphasises its limited generalisability due to small effect sizes, a lack of risk stratification in studies and significant heterogeneity in results.58 Furthermore, there are insufficient data on the optimal agents, dosing and duration of thromboprophylaxis. As a result, clinicians may feel that even higher-risk patients do not warrant routine anticoagulation. Lower rates of pharmacological thromboprophylaxis in North America may also be influenced by lower detection rates of DVT, which could reinforce the perception that routine anticoagulation is unnecessary. Further research is needed to determine whether differences in DVT surveillance contribute to regional variations in thromboprophylaxis use.
In contrast, European clinicians were more likely to prescribe pharmacological thromboprophylaxis across all risk categories, particularly for higher-risk patients. This aligns with the ESVS and NICE recommendations,1,8 which emphasise an individualised approach to VTE prophylaxis with careful consideration for those at greater risk. However, the survey also suggests that many clinicians prescribe pharmacological thromboprophylaxis even for patients who are not at an increased risk of VTE. This may reflect a lack of clear risk stratification guidance in current guidelines or concerns about medicolegal implications.24,26
These findings highlight geographical variations in practice patterns, which may be driven by differences in resource availability, clinical guidelines and local expertise.17,69 Additionally, current pharmacological thromboprophylaxis guidelines rely heavily on retrospective studies of varying quality. For instance, NICE acknowledges that its recommendations are based on very low quality evidence,70 including studies that used outdated surgical techniques such as open vein surgery, which is no longer routinely performed. These limitations highlight the need for more rigorous prospective trials to inform future guidelines.
Thromboprophylaxis by treatment modality
Treatment modality also played a role in the administration of pharmacological thromboprophylaxis. The survey suggests that patients undergoing thermal ablation were more likely to receive anticoagulation, while non-thermal techniques such as UGFS and MOCA were associated with lower rates of pharmacological thromboprophylaxis. This is interesting given that prior reports link UGFS, particularly higher volumes of foam sclerosant, to increased thromboembolic complications.46-50
Choice of pharmacological agent
Rivaroxaban was the most commonly prescribed anticoagulant across all treatment modalities, regardless of risk profile or procedure type. This aligns with a recent meta-analysis ranking rivaroxaban as the preferred anticoagulant for thromboprophylaxis following endovenous ablation due to its lower bleeding risk.71 However, rivaroxaban is not specifically licensed for post-procedural thromboprophylaxis in varicose vein surgery,72 raising questions about its off-label use in this context. Clinicians who routinely assess VTE risk were more likely to omit pharmacological thromboprophylaxis for non-thermal techniques, suggesting a more selective approach to anticoagulation based on perceived procedural risk.
Limitations
The generalisability of this survey’s results is limited due to the lack of responses from large parts of Africa, the Middle East and areas of Asia and South America. This uneven geographic distribution may be due to factors such as language barriers, varying levels of interest or expertise in the subject or limited access to the survey. These gaps could introduce the possibility of regional bias, potentially limiting the applicability of these findings to under-represented regions. Future surveys could mitigate this issue by offering translations to increase participation.
Small sample sizes in certain regions, such as Australasia, Africa, Asia and the UK, may limit the reliability of regional comparisons. Additionally, the reliance on self-reported data introduces potential selection bias. Vascular surgeons may be over-represented, and respondents may overstate adherence to guidelines. Furthermore, variability in how respondents interpreted “higher risk” patients also suggests inconsistencies in risk stratification.
The survey did not collect information on the use of the CEAP (Clinical-Etiology-Anatomy-Pathophysiology) classification or procedural indication for thromboprophylaxis decisions, despite anecdotal evidence that some clinicians incorporate these factors.73 This omission may have limited the study’s ability to capture the full scope of risk assessment practices.
Finally, while the survey presents descriptive statistics, it does not include statistical analyses to assess the significance of regional differences. The absence of confidence intervals or p values makes it unclear whether observed variations reflect true differences or random variation.
These limitations highlight the need for cautious interpretation of the results. Future research should prioritise broader geographic representation, standardised risk assessment criteria and more robust statistical analysis to strengthen the validity of findings.
Conclusions
This global survey provides valuable insight into current global practices following superficial endovenous interventions, highlighting considerable variability across regions, treatment modalities and patient VTE risk profiles. While most clinicians routinely assess VTE risk prior to these procedures, the absence of a validated risk assessment tool for this specific patient population remains a significant gap. The findings from this survey suggest that pharmacological thromboprophylaxis is more commonly used in thermal modalities than in non-thermal modalities, with rivaroxaban being the most frequently prescribed anticoagulant across regions. These results emphasise the need for clearer, evidence-based guidelines to standardise key aspects of thromboprophylaxis, including risk stratification criteria, the role of pharmacological thromboprophylaxis in this patient population and the selection of appropriate pharmacological agents. Addressing these gaps would help reduce practice variation and promote a more consistent approach to VTE prevention. High-quality randomised controlled trials, such as the ongoing THRIVE study, will be crucial in refining thromboprophylaxis recommendations and addressing current uncertainties in clinical practice. This survey’s findings should be interpreted in light of its limitations, including potential selection bias, regional disparities in response rates and reliance on self-reported data.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2025;4(2):81-93
Publication date:
February 28, 2025
Author Affiliations:
1. Academic Section of Vascular Surgery, Department of Surgery and Cancer, Imperial College London, Charing Cross Hospital, London, UK
2. Imperial Vascular Unit, Imperial College Healthcare NHS Trust, St Mary’s Hospital, London, UK
3. Academic Vascular Surgery Unit, Hull York Medical School, Hull, UK
4. Department of Vascular Surgery, Hull University Teaching Hospitals NHS Trust, Hull, UK
Corresponding author:
Alun Huw Davies
Professor of Vascular Surgery, Department of Surgery & Cancer, Imperial College London, W6 8RF, UK
Email: [email protected]











