ORIGINAL RESEARCH
Outcome of covered stents for severe aorto-iliac occlusive disease (AIOD) in patients with chronic limb-threatening ischaemia
Juszczak MT,1,2,3 Elboushi A,1,3,4 Popplewell M,1,3,4 Mohammed W,1,3 Nasr H,1,3,4 Adam D,1,3,4 Claridge M,1,3,4
Plain English Summary
Why we undertook the work: In peripheral arterial disease (PAD), the arteries fur up with thick fatty deposits resulting in less blood flowing down to the leg muscles. The disease and risk factors are like those of heart attacks and stroke. One in 50 patients with PAD develops an extreme form with severe pain at rest and gangrene; this is called critical limb-threatening ischaemia and is associated with a high risk of limb loss and death. This usually requires urgent restoration of blood flow. When the disease affects the main arteries supplying the legs (aorta and iliac arteries), restoration of blood flow requires major surgery: aorto-femoral bypass (open surgery) or aorto-iliac stent grafting (endovascular procedure). Endovascular surgery is a keyhole technique to restore blood flow through blocked arteries. It is minimally invasive and can be applied to many different parts of the body. It is a safe alternative technique to open surgery and is generally tolerated better by patients but thought to be less durable. This study looked at using this technique to insert covered stents into severely diseased aorta and iliac arteries (aorto-iliac occlusive disease; AIOD) to improve the blood supply in patients presenting with severe pain in the legs at rest and/or gangrene.
What we did: We reviewed clinical records and x-rays of patients undergoing stenting for AIOD in Birmingham Vascular Centre between 2006 and 2017. We followed up the patients looking at whether they survived, underwent an amputation, or had further surgery for the same problem.
What we found: Eighty-eight patients with severe AIOD were treated. Fifty-four had rest pain and 34 had tissue loss. The surgery had a high rate of procedural success (99.8%). Four patients died within 30 days of the procedure. Postoperative complications occurred in one in three patients. Two out of three patients were alive and eight out of 10 did not require any further intervention at three years after surgery. In seven out of 10 patients, the stents were still carrying blood at three years after the procedure. Patients with gangrene had much worse outcomes than patients with rest pain. Seven patients underwent major limb amputation during the follow-up. We found that the late amputation was not associated with patency of the stent graft or additional procedures to maintain the patency.
What this means: The technique using the simple configuration of covered stents may be a very good option for patients presenting with severe AIOD as it is less invasive than open surgery. This group of patients has a high mortality rate, particularly if presenting with tissue loss, and therefore increased durability may not be of benefit to them. Our results compared favourably with other published results including studies using more elaborate techniques to improve blood supply in AIOD.
Abstract
Objective: To determine early and medium-term outcomes in patients with chronic limb-threatening ischaemia (CLTI) due to Transatlantic Inter-Society Consensus (TASC) C/D aorto-iliac occlusive disease (AIOD) treated with covered stents (CS). Design: A retrospective single-centre case series.
Methods: Analysis of consecutive patients treated with CS for TASC C/D AIOD and CLTI between 2006 and 2017. The primary outcome was amputation-free survival (AFS). Secondary outcomes were survival, major limb amputation (MLA), patency, complications and freedom from re-intervention.
Results: Eighty-eight patients (61 men; 7 TASC C; 81 TASC D) were treated for CLTI (54 rest pain; 34 tissue loss). One hundred and twenty limbs were revascularised with 99.8% technical success. The 30-day mortality was 4.5%. Postoperative complications occurred in 30 (34%) patients. Seven (8%) patients underwent MLA. AFS was 76.0% (95% CI 65.6 to 83.6) and 66.6% (95% CI 55.2 to 75.7), primary patency 83.9% (95% CI 71.0 to 91.4) and 70.5% (95% CI 52.8 to 82.6), and freedom from re-intervention 92.4% (95% CI 83.8 to 96.5) and 83.2% (95% CI 71.2 to 90.5) at 1 and 3 years, respectively. MLA was not associated with target lesion revascularisation (OR 4.35, 95% CI 0.89 to 21.18, p=0.073) or CS patency (OR 0.33, 95% CI 0.03 to 3.43, p=0.360). Age (HR 1.10, 95% CI 1.05 to 1.14, p<0.001), creatinine (HR 1.01, 95% CI 1.00 to 1.02, p<0.001), urgency (emergency: HR 3.10, 95% CI 0.97 to 9.98, p=0.057; urgent: HR 3.71, 95% CI 1.25 to 10.97, p=0.018), tissue loss (HR 5.34, 95% CI 2.36 to 12.07, p<0.001), postoperative respiratory complications (HR 5.37, 95% CI 1.44 to 20.01, p=0.012), congestive cardiac failure (HR 0.23, 95% CI 0.05 to 0.99, p=0.049) and implantation of bilateral CS (HR 0.38, 95% CI 0.15 to 0.94, p=0.036) were independently associated with AFS.
Conclusions: Covered stents in patients with TASC C/D AIOD presenting with CLTI are associated with acceptable early and medium-term outcomes in these multi-morbid patients with reduced life expectancy. CS patency was not associated with MLA or AFS and may therefore represent a poor surrogate endpoint for assessing the efficacy of endovascular intervention for AIOD.
Introduction
The most recent iteration of the Inter-Society Consensus Document for the Management of Peripheral Arterial Disease from 2015 (Trans-Atlantic Inter-Society Consensus; TASC II) has suggested that all types of aorto-iliac occlusive disease (AIOD) may be treated using either an endovascular or open approach, provided the clinical team has sufficient expertise in the given modality, and after considering the patient’s functional status, co-morbidities, prognosis and life expectancy.1 This recommendation is supported by a recent systematic review and meta-analysis of 11 observational studies which demonstrated equivalence of open surgical and endovascular revascularisation with respect to limb salvage and patency rates, with open surgery associated with a small survival advantage (HR 0.75).2
While percutaneous transluminal angioplasty is considered appropriate for short stenotic lesions, stenting is advocated for more extensive disease. Covered stents (CS) have been shown to be associated with better freedom from re-stenosis than bare metal stents in one randomised controlled trial.3 However, a recent meta-analysis of 18 observational and one randomised study failed to demonstrate a significant difference in primary patency.4 However, an inability to accurately assess and compare the extent of the lesions between studies and the heterogeneous nature of the disease severity and clinical presentations makes it challenging to interpret such analyses.
The present study aimed to report the early and medium-term outcomes of a homogenous group of patients presenting with CLTI due to TASC-C and TASC-D AIOD who were treated with CS in a single UK vascular centre.
Methods
This case series has been reported in line with the PROCESS guidelines.5 Data were collated and the results are presented in accordance with the reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral arterial disease.6 This was a retrospective single-centre case series using anonymised, routinely collected data within a clinical audit framework; no intervention was performed, and patients were not contacted outside their routine clinical care. Therefore, specific ethical approval was not required and patients’ consent was not sought.
Patient selection
We interrogated an electronic database of consecutive patients who underwent treatment with primary CS for CLTI secondary to TASC C and D AIOD in a single UK centre between June 2006 and March 2020. Patients presenting with acute limb ischaemia and those with a history of prior endovascular or open intervention on the aorto-iliac segment were excluded. Patients were routinely assessed within a multidisciplinary team framework involving vascular surgeons, vascular interventional radiologists and vascular anaesthetists. All patients were considered to be high-risk or unfit for open anatomical bypass and a considerable majority were unsuitable for a standard radiological approach due to the need for a concomitant outflow procedure. An endovascular procedure was considered the first-line treatment in patients who were also suitable for an extra-anatomical bypass.
Endovascular procedures and follow-up
All procedures were performed by endovascular surgeons, initially in a standard operating theatre using a mobile image intensifier (OEC9900, GE Healthcare, Chalfont St Giles, UK) and, from October 2015 onwards, in a dedicated hybrid operating suite (Discovery, GE Healthcare, Chalfont St Giles, UK). Cases were planned on TeraRecon Workstation (Aquarius Intuition, TeraRecon GmbH, Germany) using vessel analysis module.
Access included open surgical femoral artery exposure or ultrasound-guided percutaneous common femoral artery puncture. If required, proximal access was achieved using right infraclavicular axillary artery exposure. Following access, patients were administered 5000 IU of intravenous unfractionated heparin. If required, common femoral endarterectomy (CFEA) and profundaplasty with an autologous or prosthetic patch was performed first and extended into the distal external iliac artery creating a landing zone above the inguinal ligament for the CS. Lesions were crossed intraluminally and/or subintimally using a combination of retrograde and antegrade approaches. The CS reconstruction was performed from distal to proximal usually using a combination of distal self-expanding GORE® VIABAHN® (W. L. Gore & Associates Inc; minimum diameter 7 mm) and proximal balloon-expandable Atrium® V12® (Maquet- Getinge AB) or GORE® VIABAHN® VBX® (W. L. Gore & Associates Inc) to ensure accurate proximal deployment. Reconstruction of the aortic bifurcation involved simultaneous balloon-expandable CS deployment at the same level in the infrarenal aorta and simultaneous post-dilatation. Since there is no high-level evidence favouring any type of stents, covered rather than bare metal stents were used in our unit based on surgeons’ preference. Although chosen due to a hypothetical risk of rupture of a stented vessel, we recognise that it was supported only by anecdotal evidence.
Patients were not enrolled into a specific CS surveillance programme but were reviewed based on presenting symptoms, the clinical picture at the time of discharge and the risk of limb loss.
Data collection and definitions
The following data were collected: demographic details, co-morbidity, American Society of Anesthesiologists Physical Status Classification System (ASA), essential medications, smoking status, anatomical and clinical grading of the disease, procedural details, perioperative complications, total hospital and critical care length of stay, patient survival, amputation and re-intervention during follow-up.
Anatomical grading of lesions was based on TASC II guidelines.7 Analysis of CT angiograms was performed by four assessors (MTJ, HN, AE and MC). Equivocal cases were resolved by consensus.8 Clinical severity was recorded using Rutherford staging criteria.
The severity of infra-inguinal disease was assessed and defined, irrespective of the level, as none (absence of stenotic or occlusive disease), moderate (presence of stenotic disease) and severe (presence of occlusive disease).
Technical success was defined as immediate angiographic patency demonstrated by post-procedural intraoperative imaging without evidence of failure (clinical or radiological) within the first 24 hours. Patency was defined as either palpable groin pulse or absence of radiologically significant (>70%) re-stenosis or occlusion in the target artery on follow-up duplex imaging. Target lesion revascularisation was defined as any procedure performed to restore luminal patency after luminal loss or to prevent luminal loss in previously treated AIOD.
The time (days) from index procedure to discharge to the patient’s usual place of residence or another healthcare provider was defined as length of hospital stay (LOS). Unplanned admission to the hospital for any reason within 30 days of discharge was considered readmission.
Outcomes
The primary outcome was the amputation-free survival (AFS). Secondary outcomes were major limb amputation (MLA), re-intervention and patency rates, incidence rate of complications, LOS during the index admission and overall survival. Survival status was verified by cross-referencing electronic patient records with the NHS-wide mortality database (Spine, NHS Digital) derived from death records from the Office for National Statistics. The amputations were verified using hospital and primary care records.
Statistical analysis
Results were analysed in pseudonymous format using R statistical environment (R version 4.0.4, R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
Continuous variables were presented as median (interquartile range; IQR) unless indicated otherwise (irrespective of outcome of normality assessment). Categorical data were presented as frequencies (proportions; %). The Student’s t-test and Wilcoxon rank-sum test were used to compare continuous data and the Pearson’s χ2 test and Fisher’s exact test were used to analyse categorical data. The Haldane–Anscombe correction was used when appropriate. Median follow-up was reported as the observed follow-up in all subjects (irrespective of outcome). The follow-up data were locked on 1 July 2020.
Overall survival, AFS and freedom from re-intervention were assessed by calculating the Kaplan–Meier product limit estimator with right-censoring of survival data. The overall patency was determined by calculating the Kaplan–Meier product limit estimator with left-censoring of patency data. The results were presented as estimates with 95% confidence intervals (95% CI) unless stated otherwise.
The effects of covariates (clinical, anatomical and procedural factors) were estimated using univariable (χ2, log-rank test) and multivariable (logistic regression and Cox proportional hazards model) analysis. Effect size was presented as hazard ratio (HR) or odds ratio (OR) with 95% CI. Purposeful selection of covariates for multivariable models was based on univariate p<0.1, data quality and clinical judgement. Missingness was treated by pairwise deletion. A p value of <0.05 was considered significant; a p value correction for multiple testing was not performed to avoid type 2 error.
Results
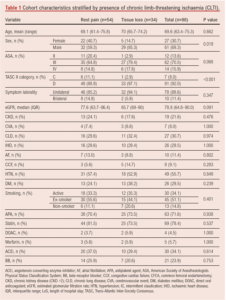
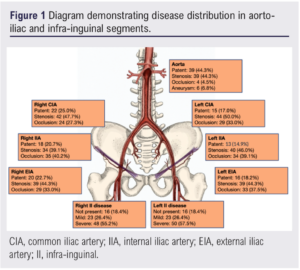
Between September 2008 and March 2020 a total of 120 limbs in 88 consecutive patients of mean age 69.6 years (range 46.8–95.4) were treated. Patient demographics and clinical characteristics are shown in Table 1 and disease distribution is shown in Figure 1.
Clinical presentation
Seventy-eight patients (89%) presented with unilateral symptoms and 10 (11%) with bilateral symptoms. Fifty-four patients (61%) had ischaemic rest pain (Rutherford stage 4) and 34 (39%) had tissue loss (Rutherford stage 5 and 6). Seven patients had TASC C lesions (8%) and 81 (92%) had TASC D lesions (Table 1).
Procedural details
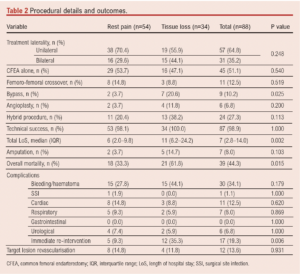
Twenty-four patients (27%) underwent elective intervention, 37 (42%) were treated urgently and 27 (31%) required emergency surgery.
Femoral access was gained with open exposure of 111 groins and percutaneous in nine.
Fifty-seven patients (65%) underwent unilateral CS implantation: two of these had bilateral symptoms but only the most symptomatic limb was treated, and one patient had a femoro-femoral crossover bypass to permit bilateral revascularisation. Thirty-one patients (35%) had bilateral CS implantation; 24 of these presented with unilateral symptoms. The aorto-iliac revascularisation procedure was technically successful in 87 patients (99%).
An outflow procedure was performed in 108 limbs (69 patients, 78%), CFEA alone in 82 limbs (45 patients, 51%) and an outflow procedure other than CFEA was performed in 26 limbs (24 patients, 27%). These data are listed in Table 2.
Complications and length of stay
The 30-day mortality was 4.5% (4/88). Peri-procedural complications occurred in 30 patients (34%; Table 2). There were 17 (19%) surgical complications which required unplanned re-intervention. The median LOS was 7.0 days (IQR 2.8–14.0). Seventeen patients spent a total of 35 days in the High Dependency Unit (HDU; level 2 care) and five patients spent 21 days in the Intensive Care Unit (ICU; level 3 care). Twelve patients (12; 14%) required unplanned readmission within 30 days of discharge.
Mortality and amputation-free survival
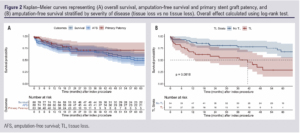
The median observed follow-up was 39.3 months (IQR 18–71.6). Forty-six patients died during the follow-up period (36.8%). The estimated overall survival was 85.6% (IQR 78.1–90.7%) at 1 year and 73.8% (IQR 64.7–80.8%) at 3 years (Figure 2A).
The median (observed) follow-up for AFS was 29.9 months (IQR 12.1–59.5). The estimated AFS was 76.0% (IQR 65.6–83.6%) at 1 year and 66.6% (IQR 55.2–75.7%) at 3 years (Figure 2A). Tissue loss was associated with inferior AFS at 3 years (51.1% (IQR 32.8–66.7%) versus no tissue loss 76.1% (IQR 61.5–85.8%); HR 2.90 (IQR 1.55–5.46), p<0.001; Figure 2B), while bilateral treatment was associated with better AFS at 3 years (77.4% (IQR 58.4–88.5%) versus unilateral 60.9% (IQR 46.3–72.6%); HR 0.46 (IQR 0.22–0.96), p=0.039).
Stent graft patency and the fate of the treated limb
Twelve patients (14.0%) required target lesion revascularisation (TLR). Five patients (6%) experienced permanent CS occlusion. The overall estimated CS patency was 96.3% (IQR 84.7–99.1%) at 1 year and 90.1% (IQR 74.7–96.4%) at 3 years, with median observed follow-up of 9.9 months (IQR 2.2–25.3).
The estimated primary patency was 83.9% (71.0–91.4%) at 1 year and 70.5% (52.8–82.6%) at 3 years (median observed follow-up 7.9 months (IQR 2–22.1); Figure 2A) and was not different for patients with or without tissue loss (HR 0.76 (95% CI 0.24 to 2.43), p=0.642). The secondary patency rates were 96.3% (84.7-99.1) and 90.1% (74.7–96.4) at 1 and 3 years.
Thirteen of 14 CS occlusions (primary patency) did not result in MLA. Seven patients (8%) underwent MLA and CS occlusion was present in only one of these patients. All MLA occurred in patients with moderate and severe concomitant infra-inguinal disease, and the majority (5/7) had TASC D AIOD.
Multivariable analysis
Major limb amputation (MLA) rate
The MLA rate was associated with diabetes (OR 9.5 (95% CI 2.3 to 38.5), p=0.002), tissue loss (OR 5.6 (95% 1.5 to 20.7), p=0.08) and re-interventions (OR 4.4 (95% CI 0.9 to 21.2), p=0.069). Multivariable analysis showed diabetes to be independently associated with limb loss (OR 6.0 (95% CI 1.1 to 46.6), p=0.048).
Amputation-free survival (AFS)
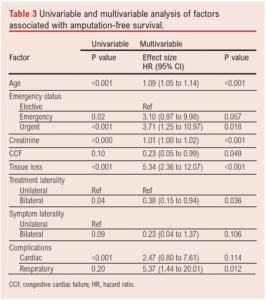
Factors associated with AFS on univariable analysis are shown in Table 3. Multivariable analysis showed that age (HR(per unit increase) 1.10 (95% CI 1.05 to 1.14), p<0.001), presence of tissue loss (HR 5.34 (95% CI 2.36 to 12.07), p<0.001), preoperative creatinine (HR(Cr per unit change) 1.01 (95% CI 1.00 to 1.02), p<0.001), non-elective status (emergency: HR 3.10 (95% CI 0.97 to 9.98), p=0.057; urgent: HR 3.71 (95% CI 1.25 to 10.97), p=0.018), congestive cardiac failure (HR 0.23 (95% CI 0.05 to 0.99), p=0.049), bilateral CS treatment (irrespective of laterality of symptoms; HR 0.38 (95% CI 0.15 to 0.94), p=0.036) and postoperative respiratory complications (HR 5.37 (95% CI 1.44 to 20.01), p=0.012) were independently associated with AFS (Figure 3, Table 3).

Discussion
The present study describes a large single-centre series of consecutive patients with CLTI and severe AIOD managed endovascularly with CS combined with an outflow procedure in the majority. The immediate technical success was high (>99%), 30-day mortality was low (4.5%) and overall AFS at 3 years was acceptable (tissue loss 51% vs rest pain 76%). CS occlusion was independent of anatomical and clinical factors (including TASC category and Rutherford staging criteria) and there was no association between MLA and TLR or CS patency. Advanced age, emergency intervention, tissue loss and postoperative pulmonary complications were independently associated with reduced medium-term survival.
While guidelines and systematic evidence reviews may, to some extent, support an endovascular-first approach for AIOD, controversy remains regarding the optimal intervention for TASC C and D lesions.1,2,9 The present study demonstrates that, at the very least, an endovascular-first approach can be justified in the majority of patients who present with tissue loss secondary to TASC C and D AIOD as the burden of co-morbidity is high, medium-term life expectancy is low (54% 3-year survival), and consequently many are unlikely to benefit from the greater durability of open surgery. Open surgery may have a continued role in patients with rest pain who have a better medium-term survival.
CS patency is an attractive hard endpoint for a research study and may provide useful information on technical aspects of the procedure as well as being a marker of a functional graft surveillance programme. In the present study, CS occlusion was clinically inconsequential in most patients and neither patency nor TLR were associated with limb preservation. Most of the literature on CS in patients with severe AIOD uses patency as the primary or surrogate outcome without examining its relationship with limb salvage, which is the main therapeutic objective and the most important clinical outcome in patients with CLTI.2,10 The lack of association between MLA rate and patency undermines the validity of CS patency as the primary or surrogate outcome in patients with CLTI. CS patency may, however, be an appropriate outcome measure in patients with intermittent claudication.
AIOD extending to involve the infrarenal aorta has traditionally been managed by aorto-bifemoral bypass grafting. Endovascular revascularisation can be performed with CS in a double-barrelled or kissing configuration (DBCS) or by using a single large aortic CS and bilateral iliac CS extensions (Covered Endovascular Reconstruction of Aortic Bifurcation, CERAB).10 Taeymans et al described 130 patients treated with CERAB over a 7-year period, the majority of whom had TASC D lesions (89%), but only one-third had CLTI, 19% required common femoral artery endarterectomy and none required an outflow procedure.11 Saratzis et al described CERAB in 116 patients treated over 8 years in six UK centres with less than half treated for CLTI.12 It is difficult to compare the outcomes of the present study with the publications on CERAB when the major indication for treatment was intermittent claudication. In patients with CLTI, the most clinically relevant and directly comparable outcome is the MLA rate, and this was not significantly different between the study by Taeymans et al and the present study: four of 42 (10%) patients at median 24 months follow-up after CERAB compared with four of 31 (12.5%) who had DBCS in the present series at median 37 months follow-up (OR 0.71 (95% CI 0.12 to 4.19), p=0.72). Based on presented results, the simpler DBCS configuration appears to be as effective as CERAB for limb salvage in CLTI. However, CERAB has been shown to have a different flow pattern to other CS configurations. If this were to have a positive effect on CS patency, this may confer clinical advantage to patients treated for intermittent claudication where greater durability is key to improving quality of life.13
The present study cohort was relatively homogeneous in terms of TASC category and clinical presentation, and this makes it difficult to interpret the findings compared with previous studies which describe small numbers of patients who are invariably heterogeneous in terms of clinical presentation (with the majority having intermittent claudication), disease severity and the requirement for outflow reconstruction.14-16
The limitations of the present study include its retrospective nature and the relatively small numbers of patients treated over a long period where practice inevitably changes. Another potential weakness of the study was a lack of standardised imaging-based follow-up. This may make the analysis of TLR difficult. However, standardised imaging in follow-up does not seem to be supported by any evidence and, as such, is not required. We also argue that TLR may not be associated with the main composite outcome, and hence would defy the purpose of standardised/protocolised surveillance.
In conclusion, the present study demonstrates acceptable limb salvage when CS are used to treat advanced AIOD causing CLTI. The fate of the limb was not determined by the CS patency, and this does not appear to be a useful outcome measure in patients with CLTI.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2023;2(3):141-148
Publication date:
March 14, 2023
Author Affiliations:
1. Department of Vascular Surgery, Heartlands Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
2. Institute of Inflammation and Ageing, University of Birmingham, UK
3. West Midlands Vascular Research and Innovation Consortium (VaRICS), UK
4. Department of Vascular Surgery, Zagazig University Hospitals, Egypt
Corresponding author:
Martin Claridge
Department of Vascular Surgery, Birmingham Heartlands Hospital
University Hospitals Birmingham NHS Foundation Trust, Birmingham B9 5SS, UK
Email: martin.claridge@ uhb.nhs.uk











