COHORT STUDY
Prognostic value of simple frailty and malnutrition screening tools for determining surgical risk in patients with chronic limb threatening ischaemia undergoing major vascular surgery: a retrospective cross-sectional study
Palmer J,1 Pymer S,1 Smith GE,1 Ingle L,3 Harwood AE,2 Chetter IC1
Plain English Summary
Why we undertook the work: For patients with severe circulation problems to the legs, surgery is often needed. However, these patients can suffer poor outcomes following surgery. If a patient is also frail or malnourished, their outcomes are often even worse. Several tools exist to measure frailty and malnourishment, but they are sometimes complicated and not always suitable for patients with severe circulation problems to the legs, especially if they require walking or physical activity. Other tools, which are simpler to apply, may be more suitable. The aim of this study was to see if these simple frailty and nourishment tools can be used to identify patients with severe circulation problems to the legs who are more likely to have poor outcomes.
What we did: We looked at patients with severe circulation problems to the legs who had surgery. We used two frailty and two nourishment tools to identify which patients were frail and/or malnourished. We then looked at the number of patients who had died, had complications, went back to theatre or had to return to hospital after discharge. We also looked at how long patients stayed in hospital. We then looked at whether people with frailty or malnourishment were more likely to experience these things.
What we found: We included a total of 139 patients. Those who were frail were more likely to die within a year, have complications and return to hospital after discharge. Malnourishment did not increase the risk of dying within a year.
What this means: All patients with severe circulation problems to the legs who are having surgery should be assessed for frailty. Those who are frail should have specific plans to minimise their risk of poor outcomes.
Abstract
Background: This study aims to explore the prognostic value of simple frailty and malnutrition tools for determining postoperative outcomes at 30 days and one year in patients with chronic limb threatening ischaemia (CLTI) undergoing major surgery.
Methods: This was a single-centre retrospective observational cross-sectional study in a tertiary UK Vascular Centre. We applied the Derby Frailty Index (DFI), the Acute Frailty Network Criteria (AFNC), the Prognostic Nutritional Index (PNI) and the Geriatric Nutritional Risk Index (GNRI) to routinely collect clinical audit data recorded from a combination of medical and nursing notes, blood test results and discharge letters.
Results: We included 73 patients who had undergone amputation (mean age 69 (range 57–77) years; 71% male; mean body mass index (BMI) 27 (range 23–31) kg/m2) and 66 patients who had undergone bypass surgery (mean age 73 (range 64–81) years; 70% male; BMI 26 (22–30) kg/m2). The 30-day mortality rate of a frail patient (DFI and AFNC, respectively) was 21% (n=8/39) and 23% (n=6/26) compared with 7% (n=7/100) for non-frail patients. Frailty was associated with 30-day (DFI: odds ratio (OR) 3.4 (95% CI 1.2 to 10.2), p=0.027; AFNC: OR 3.4 (95% CI 1.1 to 10.7), p=0.034) and one-year (DFI: OR 7.95 (95% CI 3.4 to 18.6), p<0.001; AFNC: OR 5.9 (95% CI 2.4 to 14.7), p<0.001) mortality. Frailty, according to the AFNC, was also associated with an increased risk of re-admission. Neither of the malnutrition screening tools was associated with 30-day or one-year mortality (p>0.05).
Conclusions: Our analysis confirms that both the DFI and AFNC tools identify patients with CLTI who are at the greatest risk of poor outcomes following major surgery. Frailty warrants further investigation and should be part of the consent and joint decision-making process in this patient population, to personalise care and minimise the risk of poorer outcomes.
Introduction
Chronic limb threatening ischaemia (CLTI) is a severe manifestation of peripheral arterial disease (PAD), characterised by intractable rest pain with or without tissue loss in the form of ulceration and/or gangrene or infection.1 Approximately 1% of all PAD cases are due to CLTI and first-line treatment includes revascularisation, primary amputation or, in some cases, conservative management.1,2 In the UK in 2022 there were 9,592 lower limb revascularisations (angioplasty or bypass) and 2,233 amputations performed for CLTI.3 However, surgery often results in a poor prognosis with high 3-year mortality (37%), re-intervention (57%) and major amputation (12%) rates.4
Patients with CLTI are often older and present with multiple comorbidities.5 This carries an increased risk of morbidity and mortality after surgery and makes them more likely to be frail. Frailty is a state of vulnerability resulting in a poor recovery of homeostasis after a stressor event, triggering disproportionate changes in health status.6 Recent evidence has shown that the presence of frailty is associated with worse outcomes for patients undergoing vascular surgery, including those with CLTI.7 However, some of the applied frailty tools may not be suitable for patients with CLTI as they require an assessment of mobility and function. The Clinical Frailty Scale, which is the most widely adopted tool in vascular surgery,8 does not require such assessments. However, the criteria are open to interpretation, leading to ambiguity in the frailty assessment. Other widely used tools such as the electronic frailty index can be overly complex. Therefore, the application of simple, less ambiguous tools that only require routinely collected information and no training to apply, such as the Derby Frailty Index (DFI) and the Acute Frailty Network Criteria (AFNC) may be more appropriate.
In addition to frailty, the state of malnourishment increases surgical risk. In patients with CLTI undergoing endovascular therapy, certain characteristics (body mass index (BMI) <18.5 kg/m2) and/or serum albumin <3.0 g/dL) are associated with poorer surgical outcomes.9 The Geriatric Nutritional Risk Index (GNRI) is a simple and validated tool which considers these factors in its assessment.10 As stature and BMI may not be easily determined in some patients with CLTI, the Prognostic Nutritional Index (PNI), which has been developed and used in other clinical and surgical populations,11,12 may be a good alternative for the CLTI population.
These frailty and malnutrition tools have not been previously applied to patients with CLTI, meaning there is a dearth of evidence, despite evidence in the heart failure population.12 Application of these tools can help to identify the most vulnerable patients, facilitating a more rigorous approach to reducing surgical risk and anaesthetic management.
Therefore, the aim of this study was to explore the prognostic value of simple frailty and malnutrition tools for determining postoperative outcomes at 30 days as well as one-year mortality in patients with CLTI undergoing surgery.
Methods
Study design
A single-centre retrospective observational cross-sectional study (January to December 2016) was undertaken in a tertiary academic vascular unit in the UK, reported in line with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (see Appendix 1 online at www.jvsgbi.com).13 The study was registered with the local audit governance department (ID: 2018.047) as it did not require full ethical approval.
Study participants
Participants were >18 years of age, had undergone either a bypass or amputation procedure for CLTI and were identified via a locally maintained database. Patients who had undergone these procedures for other indications were excluded. CLTI was defined as rest pain, ulceration and/or gangrene as assessed by a vascular consultant or registrar. All patients had a full medical history, physical examination and blood tests following admission. BMI, social situation, mobility, falls risk and risk of malnutrition were recorded from data obtained on admission. All data were collated from a combination of medical and/or nursing notes, haematological results and discharge letters.
Frailty indices
Two frailty indices were used, as applied in the heart failure population.12
1. The DFI (scored as frail or non-frail) identifies frailty based on meeting one of the following criteria:
• Age >65 years and a care home resident;
• Age >75 years with confusion, falls, reduced mobility or a combination of these; or
• Age >84 years with >4 comorbidities.
2. The AFNC (scored as frail vs non-frail) identifies frailty as meeting one of the following criteria:
• Age >85 years; or
• Age 6>5 years with:
– cognitive impairment
– resident in a care home
– history of fragility fractures
– Parkinson’s disease
– recurrent falls.
Malnutrition scores
Two malnutrition indices were used:
1. The GNRI, calculated using the formula: 1.489 x albumin (g/L) + 41.7 x current weight/ideal weight and classified as follows:
• >98 = not malnourished
• 92–98 = mild malnourishment
• 82–91 = moderate malnourishment
• <82 = severe malnourishment.10
2. The PNI, calculated using the formula: 10 x serum albumin (g/L) + 0.005 x total lymphocyte count (109 cells/L) and classified as follows:
• >38 = normal
• 35–38 = moderate malnourishment
• <35 = severe malnourishment.11
Outcomes
The primary endpoint was 30-day mortality. Other outcomes included one-year mortality, postoperative complications (defined as the occurrence of any complication within 30 days), re-admissions (defined as the requirement to be re-admitted to hospital within 30 days), return to theatre rates (defined as the requirement to return to theatre within 30 days) and length of hospital stay (LoS).
Statistical analysis
Due to the nature of this study, a formal sample size calculation was not performed. All patients undergoing an amputation or bypass procedure for CLTI during the study period were eligible. Descriptive data are presented as mean and standard deviation, median and interquartile range (IQR) or count and percentages as appropriate. A χ2 test or Fisher’s exact test was used for exploring associations and an independent t-test or Mann–Whitney U test for differences between groups, as appropriate. Frailty and malnutrition indices were explored using univariate binary logistic regression for patients who died (at 30 days or one year), had postoperative complications, returned to theatre and/or were re-admitted within 30 days. The type of operation (bypass or amputation) and 30-day mortality, one-year mortality, re-admissions and return to theatre rates were not associated (p>0.05), therefore data were amalgamated and then modelled for the frailty and malnutrition scores with goodness of fit assessed using the area under the curve (AUC). Postoperative complications and the type of procedure (bypass and amputation) were modelled independently (p<0.05). A Kaplan–Meier survival curve with log rank test was used to explore mortality at one year for frail versus non-frail patients. Finally, agreement between the two frailty tools was assessed using Cohen’s kappa statistic. Data were analysed using IBM SPSS V24.0; statistical significance was accepted if p<0.05 and missing data were treated as missing and were not imputed.
Results
Baseline
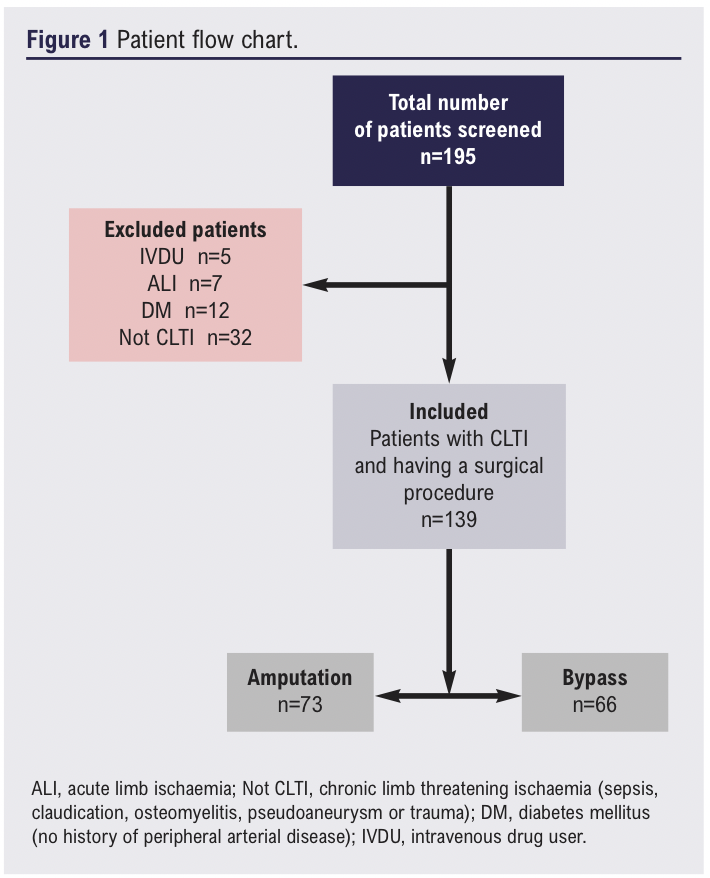
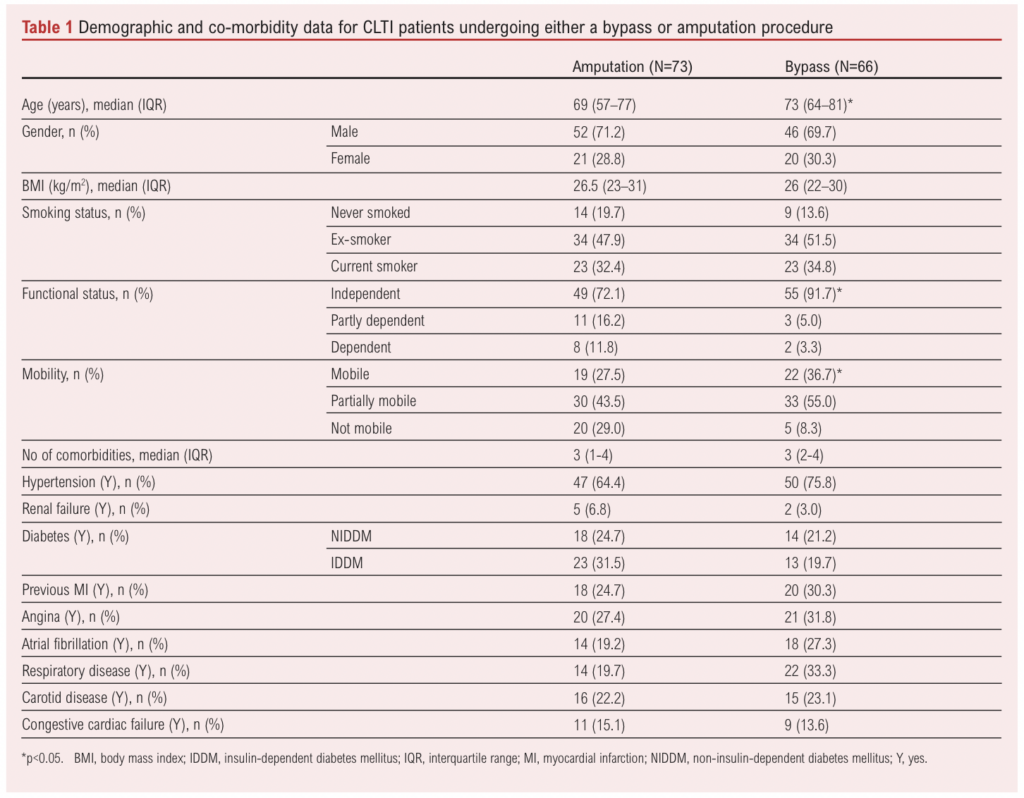
A total of 195 patients who had undergone lower limb bypass or major lower limb amputation over the study period were identified and screened for inclusion. Fifty-six patients undergoing amputation or bypass for reasons other than CLTI (including sepsis, intermittent claudication, osteomyelitis, pseudoaneurysm or trauma) were excluded (Figure 1). The remaining 139 patients (median age 70 (IQR 61–79) years, 71% male) were confirmed to have CLTI, met the inclusion criteria and were therefore included in the analysis, with 73 undergoing major amputation and 66 a bypass procedure. Baseline characteristics for type of procedure and frailty status are shown in Tables 1 and 2, respectively. Patients who underwent a bypass procedure were four years older than those who had an amputation (p=0.011), whilst patients who had an amputation were less mobile (p=0.012) and were more dependent on others for activities of daily living (p=0.018).
Frailty status
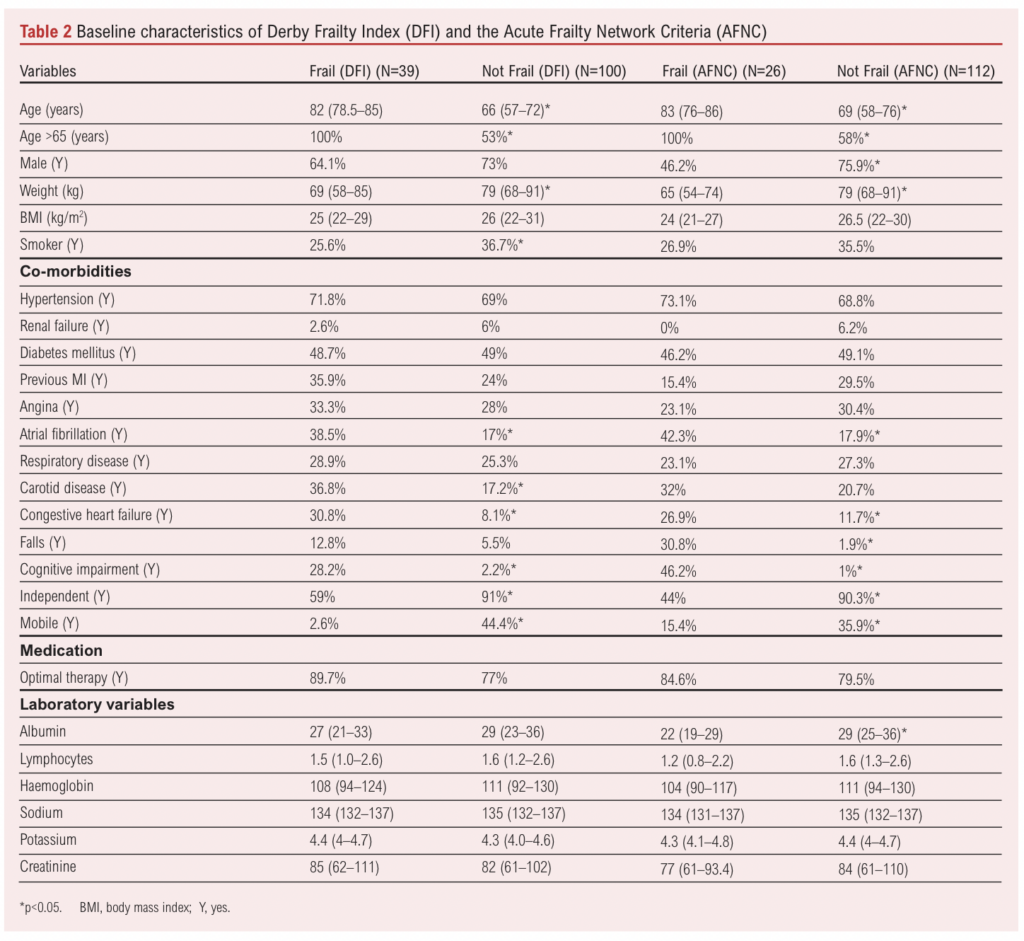
Overall, 28% (n=39/139) of patients were identified as frail using the DFI and 19% (n=26/138) using the AFNC (p<0.001). AFNC data were missing for one patient due to a lack of recording of cognitive status. For amputation and bypass procedures, the proportion of frail patients was similar for both the DFI (p=0.348) and the AFNC (p=0.327). There was moderate agreement between the two frailty tools assessed using Cohen’s kappa interrater reliability statistic (0.503, p<0.001). For both tools, the proportion of patients who were mobile without the use of any aids or were independent was significantly lower for frail compared with non-frail patients (p<0.001).
Malnutrition status
In total, 50% (n=69/137) of patients were considered malnourished according to the PNI and 68% (n=79/116) were considered malnourished according to the GNRI (p=0.001). Missing data for the GNRI were due to no height measurement (n=23), and lymphocyte count was not available for the PNI assessment (n=2). For amputation and bypass procedures, respectively, the proportion of malnourished patients was similar for the GNRI (57% vs 43%; p=0.1) but was significantly different for the PNI (75% vs 25%; p<0.001).
Relationship between frailty and malnutrition tools
The frailty tools demonstrated a concordance of 82%, with both classifying 15% (20/138) of the same patients to be frail and 67% to be not frail. Overall, the DFI identified 28% (39/139) as frail compared with 19% (26/138) using the AFNC tool (p<0.001).
The nutritional tools demonstrated a concordance of only 66%. Overall, the PNI identified 52% (n=59/114) as malnourished compared with 68% (n=78/114) with the GNRI (p=0.001). There was no association between the DFI and the PNI or GNRI (p=0.534 and p=0.960, respectively) or the AFNC and the PNI or the GNRI (p=0.430 and p=0.110, respectively).
30-Day outcomes
Mortality
Overall, 30-day mortality was 11% (n=15/139). Causes of death included pneumonia (n=4), sepsis (n=5) and myocardial infarction (n=6). Of the patients who died, 13 (87%) were either frail (n=4), malnourished (n=4) or both (n=5). Although 30-day mortality was higher in the amputation group (14%, n=10/73) than in the bypass group (8%, n=5/66), this difference was not statistically significant (p=0.245).
In the frail group, 30-day mortality ranged from 21% to 23%, depending on the tool used. Frailty, regardless of the tool used, was associated with 30-day mortality (DFI: OR 3.4 (95% CI 1.2 to 10.2), p=0.027; AFNC: OR 3.4 (95% CI 1.1 to 10.7), p=0.034). The goodness of fit (AUC) for the frailty tools was 0.642 and 0.619, respectively. However, when broken down by procedure, frailty and 30-day mortality were significantly associated in the bypass group (p=0.002) but not in the amputation group (p=0.700). Neither of the malnutrition tools was associated with 30-day mortality (PNI: p=0.254; GNRI: p=0.730).
Postoperative complications
Postoperative complications occurred in 39% of cases (n=54/139; Table 3) but were significantly lower following an amputation compared with a bypass procedure (30% vs 52%; p=0.027). Neither of the frailty indices was significantly associated with postoperative complications following an amputation (DFI, p=0.402; AFNC, p=0.913). However, frailty identified using the DFI (but not the AFNC, p=0.462) was associated with postoperative complications following a bypass procedure (OR 8.5 (95% CI 2.4 to 29.8), p=0.001). The goodness of fit (AUC) for the DFI tool was 0.869. Neither of the malnutrition tools was associated with postoperative complications following amputation (PNI: p=0.214; GNRI: p=0.869) or bypass (PNI: p=0.722; GNRI: p=0.506).
Re-admissions
Re-admission within 30 days occurred in 6% (n=8/139) of patients, with no significant difference between patients undergoing amputation (7%, n= 5/73) or bypass (5%, n=3/66, p=0.721). Frailty established using the AFNC (but not the DFI, p=0.843) was significantly associated with re-admission within 30 days (OR 4.9 (95% CI 1.1 to 21.1), p=0.033) whereas neither of the malnutrition tools was significantly associated with re-admission within 30 days (PNI: p=0.253; GNRI: p=0.938). The goodness of fit (AUC) for the AFNC tool was 0.665.
Return to theatre rates
In total, 10% (n=14/139) of patients were required to return to the theatre within 30 days. There was no significant difference in return to theatre rates between amputation (6%, n=4/73) and bypass procedures (15%, n=10/66; p=0.058). Neither of the frailty (DFI: p=0.563; AFN: p=0.647) or malnutrition tools (PNI: p=0.792; GNRI: p=0.910) was associated with returning to theatre within 30 days.
Length of stay (LoS)
The median LoS for all patients was 14 (IQR 9–25) days. Patients undergoing amputation procedures (17 (IQR 12–27.5) days) had a significantly longer LoS than those undergoing bypass procedures (13 (IQR 7–21) days; p=0.010). There were no significant differences in LoS between frail and non-frail patients using either the DFI (p=0.388) or AFNC (p=0.626) or between malnourished and nourished patients using the PNI (p=0.050). However, according to the GNRI, malnourished patients had a significantly longer LoS (16 (IQR 10–30) days) compared with nourished patients (12 (IQR 8–18.5) days; p=0.039). This difference was not driven by the difference in LoS between procedures (amputation malnourished vs nourished LoS: p=0.369; bypass malnourished vs nourished LoS: p=0.126).
One-year mortality
One-year mortality was 26% (n=36/139) with no significant difference between amputation (27%, n=20/73) or bypass procedures (23%, n=15/66; p=0.526).
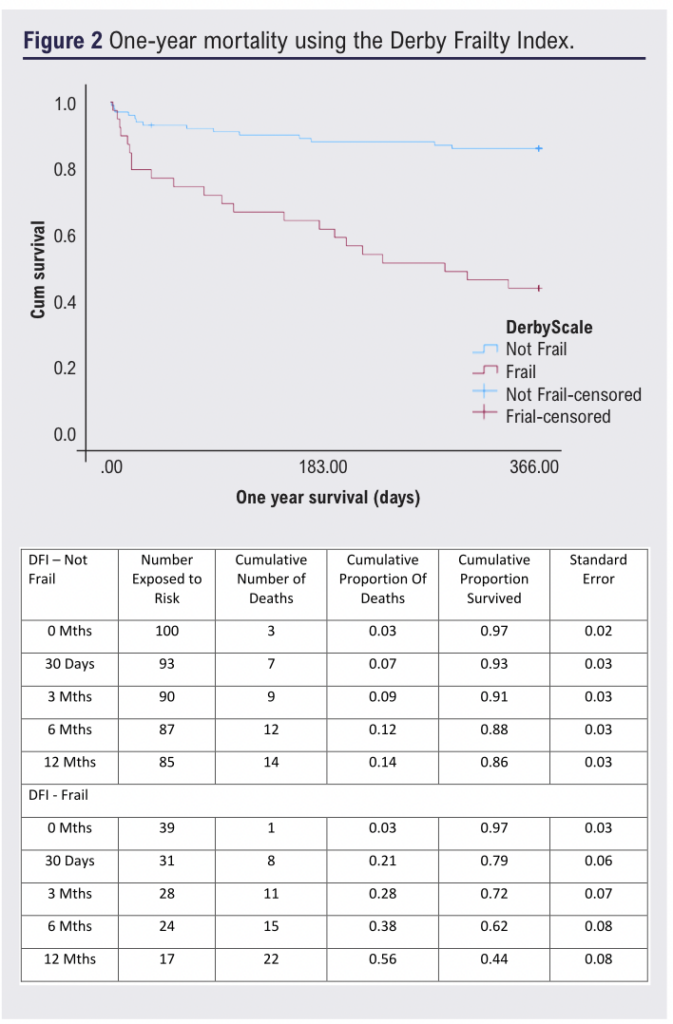
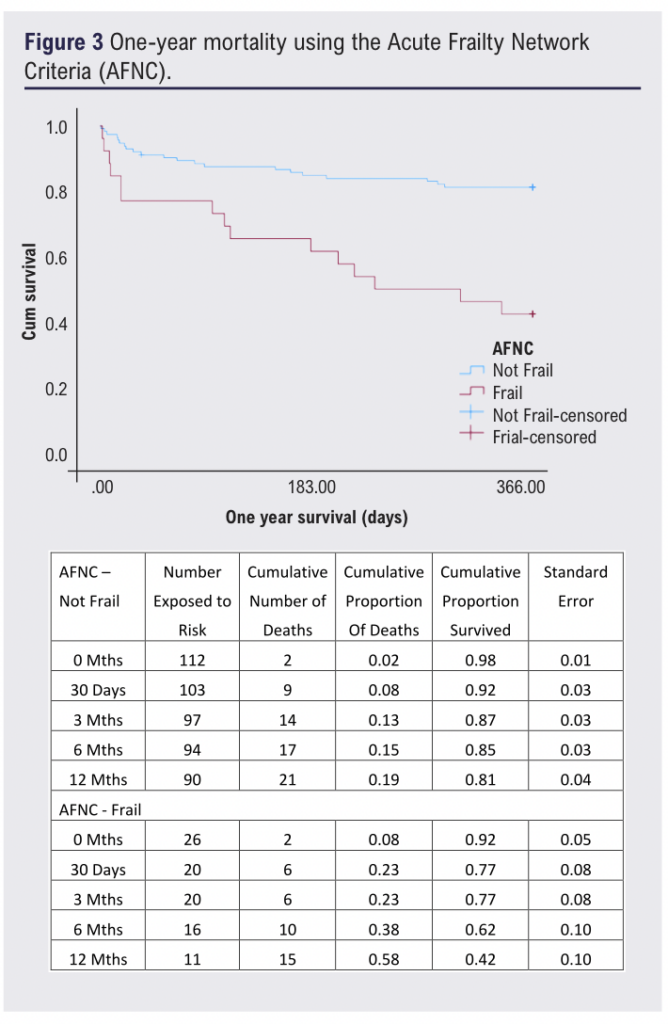
Patients considered frail by either tool had significantly higher mortality at one year, ranging from 56% to 58% (p<0.05; Figures 2 and 3). Frailty was significantly associated with one-year mortality (DFI: OR 7.95 (95% CI 3.4 to 18.6), p<0.001; AFNC: OR 5.9 (95% CI 2.4 to 14.7), p<0.001; Figures 2 and 3), whilst malnutrition was not (PNI: p=0.884; GNRI: p=0.177). Frailty and one-year mortality were significantly associated in the bypass (p<0.001) and amputation groups (p=0.002). The goodness of fit (AUC) for the frailty tools was 0.723 and 0.654, respectively.
Discussion
This study aimed to assess how frailty and malnutrition, identified using simple to apply, less ambiguous tools, are associated with outcomes in patients with CLTI undergoing lower limb vascular surgery. We have demonstrated that frailty and malnutrition are prevalent in this population and that frailty is strongly associated with both 30-day and one-year mortality, especially in patients undergoing a bypass procedure. Frailty is also associated with postoperative complications and re-admissions. Overall, it appears that both tools are able to strongly describe the risk of poorer outcomes following surgery. These findings mirror those reported previously in that frailty, assessed using more widely adopted tools such as the Clinical Frailty Scale and the Modified Frailty Index, is associated with mortality and morbidity, but not length of stay, for patients with PAD undergoing revascularisation.12,14-16
This therefore strengthens the need to consider frailty in general in patients being considered for major vascular surgery, particularly in the joint patient and clinician decision-making process. It also provides new evidence for the utility of alternative tools, the DFI and AFNC, which hitherto have not been considered in patients with CLTI, despite evidence in the heart failure population.12 This suggestion that frailty needs to be considered in the joint decision-making process is furthered when considering the results recently reported for a study exploring frailty in patients with CLTI presenting to a vascular limb salvage clinic. This study demonstrated that, on multivariable analyses, frailty was not independently associated with death or amputation.17 However, frail patients were more likely to be managed conservatively, suggesting that, for a select group of frail patients, conservative management may lead to better outcomes than aggressive invasive treatment. This warrants further exploration to identify which characteristics contributing to frailty make conservative management more appropriate.
One interesting finding is that frailty was only associated with 30-day mortality following bypass but not following amputation, and this association was stronger when using the DFI, as evidenced by the greater AUC. Additionally, frailty established using the DFI was associated with postoperative complications, again only in the bypass group. This suggests that the DFI is able to identify patients who are at an increased risk of complications following bypass procedures and these complications may also be contributing to the increased risk of 30-day mortality in this group. Therefore, any patient with CLTI undergoing a bypass procedure who is identified as frail according to the DFI requires appropriate input and optimisation across the perioperative pathway to reduce their risk of postoperative complications and consequent 30-day mortality.
When considering the AFCN tool, frailty was associated with an increased risk of re-admission. This may be secondary to the fact that, according to this tool, patients aged >85 years are considered frail, regardless of any other factors. This notion is supported by a study in a similar patient cohort which demonstrated that patients aged >77 years were at an increased risk of multiple re-admissions.18 However, although frail patients were significantly older than their non-frail counterparts, they also differed in terms of the incidence of important co-morbidities and a combination of age and co-morbidities is more strongly associated with poorer outcomes than chronological age alone.19 This suggests that there may be other drivers than age which contribute to the increased risk of re-admission. It is therefore important that non-frail elderly patients are not denied treatment based on age alone.
The incidence of malnutrition in our patient cohort significantly differed depending on the screening tool used. The proportion of patients identified as malnourished using the PNI, which has not been previously applied to vascular patients, was similar to that demonstrated in heart failure patients.12 Conversely, the proportion of patients identified as malnourished using the GNRI differed substantially to previous studies.20,21 However, these studies did not consider those with mild malnourishment as malnourished. When the same criteria are applied to our cohort, the proportion of malnourished patients is similar.20,21 Regardless, our findings do differ from those in vascular20,21 and other populations,12 as neither tool was associated with postoperative outcomes. This suggests that, although important, nutritional status may not be as relevant as frailty for patients with CLTI undergoing major surgery.
The finding that these malnourishment tools are not associated with postoperative outcomes is, however, more likely to be related to the inflammatory process which influences lymphocyte cell count and the synthesis of serum albumin. Indeed, the recent ASPEN position paper specifically states that serum albumin characterises inflammation, rather than describing nutritional status.22 Given that CLTI is a systemic inflammatory condition, the application of these tools, which are heavily weighted by albumin concentration, may not be appropriate. An example of how these tools may be influenced by inflammation is provided within our data. The PNI, which does not consider other physical patient characteristics in its assessment, characterised a significantly greater proportion of amputation patients as malnourished compared with bypass patients. This is likely to be related to the elevated inflammatory state of patients undergoing amputations compared with that of those undergoing bypass procedures.
Clearly, further work considering malnourishment in patients with CLTI undergoing surgery is required. Such studies should adopt appropriate tools which do not consider serum albumin in their assessment, such as those highlighted in the recent ASPEN position paper.22
Implications for practice and future directions
Frailty assessment allows evidence-based quantification of risk during the consent process and should become mandatory. These simple tools can be applied prior to ward rounds or clinic appointments as they use routinely collected data and require no additional training. Identifying frailty provides an opportunity to work with the multidisciplinary team to optimise the patient and plan admission with access to high dependency wards as well as appropriate rehabilitation and discharge. However, there is a need for consensus on the most appropriate frailty tool for use in patients with CLTI. This study suggests that either the DFI or the AFNC may be appropriate, although the DFI may be more appropriate in patients undergoing bypass, evidenced by its ability to predict postoperative complications which may be contributing to 30-day mortality. However, before we can recommend their use, these tools warrant further investigation to explore their validity, reliability, responsiveness, patient acceptability and clinical functionality as well as how they compare with more widely adopted tools such as the Clinical Frailty Scale.
Additionally, although factors relating to frailty are often unmodifiable, frailty is associated with poor physical function23 and depleted physiological reserve, so it may identify a specific group of patients who could benefit from ‘prehabilitation’. Evidence suggests that prehabilitation combined with rehabilitation improves postoperative outcomes in other surgical specialities24,25 and thus warrants further investigation in frail patients with CLTI. It is, however, important to consider this in the context of the current PAD-QIF targets for revascularisation, which are 5 days for inpatients and 14 days for outpatients.26 Therefore, prehabilitation, if considered in future investigations, will need to be intensive to maximise the limited timeframe available. If prehabilitation is not feasible, an alternative could be the use of a comprehensive vascular geriatric service, with the inclusion of geriatric experts. Recent evidence suggests a service such as this can improve outcomes and reduce 30-day mortality in frail vascular inpatients.27
Finally, malnutrition, whilst clearly important, was not associated with poorer outcomes in this study. Further work is required to determine a reliable malnutrition tool for patients with CLTI.
Limitations
This study is not without limitations. First, the study is retrospective with a limited sample size drawn from a single centre. This limits generalisability and increases the possibility of a type II error. In addition, confounders, both known and unknown, could not be controlled for. Furthermore, the data collection period (2016) is now eight years ago and it is possible that the findings presented here may not be reflected in current practice with the more recent developments in perioperative and ward level care. However, the results are still relevant and this study acts as a fundamental starting point to stimulate further prospective, appropriately designed trials considering the impact of frailty, identified using these simple-to-apply tools, and malnutrition, using other appropriate tools, in contemporaneous cohorts of patients with CLTI.
One final limitation is the lack of available data on CLTI severity in the form of Rutherford classifications, WIfI classifications and haemodynamic measures. Due to the nature of this study and its retrospective data collection, we were unable to obtain this information despite the likelihood of it being recorded. Therefore, it is imperative that future prospective studies collect and report these data.
Conclusion
Frailty is associated with an increased risk of worse outcomes for patients with CLTI undergoing major surgery. Frailty should be considered routinely in this patient cohort to inform the consent and joint decision-making processes, to personalise care and to minimise the risk of poorer outcomes, although further research is needed.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(3):122-130
Publication date:
May 22, 2024
Author Affiliations:
1. Academic Vascular Surgical Unit, Hull York Medical School, Hull, UK
2. Department of Sport & Exercise Sciences, Institute of Sport, Manchester Metropolitan University, Manchester, UK
3. Department of Sport, Health & Exercise Science, University of Hull, Hull, UK
Corresponding author:
Sean Pymer
Academic Vascular Surgical Unit, Tower Block, Hull Royal Infirmary, Hull HU3 2JZ, UK
Email: [email protected]











