ORIGINAL RESEARCH
Understanding variation in the management of AAA in the UK: composition and function of multidisciplinary team meetings and information resources provided to patients
Winterbottom A,1 Bekker HL,2 Dhesi J,3 Hammond C,4 Howell S,5 Saratzis A,6 Birmpili P,7 Ruffell L,8 Richards SH9
Plain English Summary
Why we undertook the work: The ‘aorta’ is the largest blood vessel in the body. It starts at the heart and passes through the chest and tummy. Over time, the aorta can become bigger and weaker. When this happens, a person may have an abdominal aortic aneurysm (AAA). People do not normally feel unwell, but the aorta may burst and cause bleeding inside the body and death. If an AAA is diagnosed and the aorta reaches a certain size, a person can have an operation to repair it. This reduces the risk of the AAA bursting. The NHS performs about 6,000 AAA repairs each year in hospitals across the UK. To help doctors decide how to treat AAA, national guidelines are available. Despite this, there are large differences between hospitals in how AAAs are repaired, including who is offered treatment and the type of treatment that is carried out. These differences are not because of differences between patients (eg, age, sex, ethnicity). They suggest that medical teams have different ways of making decisions about who should have treatment for an AAA and how the treatment is carried out.
What we did: This study described how different medical teams at different hospitals make decisions about treating patients with AAA. Seventeen doctors (24% of centres) leading AAA care completed a questionnaire about how their service organises and delivers care. We used this information to describe how AAA care varies between hospital teams and explore how this might lead to different treatments offered to patients.
What we found: All centres hold multidisciplinary team (MDT) meetings with health professionals from different specialties. However, there were differences in who attended, and how and when the meetings occurred. Clinicians also reported differences in how they presented information about risk, how they ask patients about their preference for treatment, and how someone is managed if they are not suitable for surgery. The written patient information leaflets did not describe the ‘non-surgical’ option adequately.
What this means: The survey shows differences in how people with AAA are prepared and managed across UK vascular centres. Including the wider healthcare team, improving the way in which risk is presented to patients and defining a non-surgical pathway for those unsuitable for surgery may help improve consistency between hospitals for the management of people with AAA.
Abstract
Objective: Variation in abdominal aortic aneurysm (AAA) repair practice is reported nationally. This may be due to gaps in the evidence supporting clinical decision-making, historical preferences in repair practices within centres, and variation in decision-making in multidisciplinary team (MDT) meetings. This study aims to understand the reasons behind variation in AAA repair practices in UK NHS vascular centres in terms of MDT discussions, written patient information and patient involvement in decision-making.
Design: An observational, cross-sectional organisational survey of NHS vascular centres. Methods: Consultant vascular surgeons at 50/72 UK centres were invited to participate in a researcher-administered survey. Centres were categorised using 2022 National Vascular Registry (NVR) dataset into low versus high endovascular aneurysm repair (EVAR) rates and low versus high rates of MDT review; the sample was stratified to achieve balance across the four groups. The survey captured centre characteristics, individual clinical decision-making practices, integration of patient perspectives within MDT decision-making and information provision.
Results: Seventeen clinicians completed the study (24% of centres). All centres hold MDT meetings but differ in composition, skills mix, remit and delivery. Variation was observed in how clinicians present risk information, elicit patient preferences and manage someone not deemed suitable for (or declining) repair. Written information given to patients to supplement consultations does not adequately describe the conservative management option.
Conclusions: This survey highlights variation in preparation and management of people under consideration for AAA repair across UK vascular centres. Improving input of other specialties, improving the presentation of risk to patients and defining active, non-surgical ‘conservative management’ pathways may help to improve the consistency of practice between vascular centres.
Introduction
There is marked national1,2 and international3 variation in abdominal aortic aneurysm (AAA) repair practice and, more specifically, in the proportion of patients undergoing open surgical repair (OSR), endovascular aneurysm repair (EVAR) or no repair (conservative management). In 2022, the UK National Vascular Registry (NVR) reported that 59% of 2,744 patients undergoing repair of infrarenal AAA had an image-guided minimally invasive EVAR. Rates per centre ranged from 22% to 97%.1,2 For 1.2 million men screened via the national AAA screening programme (NAAASP) with an AAA that required consideration for elective repair from 2009 to 2016, decisions regarding patient suitability for repair varied between centres, with ‘turndown rates’ – that is, those not deemed suitable for (or declining) intervention – of between 2% and 22%.1 These datasets identify significant regional variation in repair practice which cannot be explained by patient characteristics or case mix.1,2
Variation in AAA management may relate to a variety of reasons including gaps in the evidence base, historical preferences in repair practice and differences in the composition and function of multidisciplinary team (MDT) meetings.4 MDT meetings are recommended to promote good quality clinical decision-making about AAA management within vascular surgery.5 In 2022, an estimated 84% of patients with AAA were discussed at an MDT meeting.2 As a minimum, the AAA MDT should include surgeons, interventional radiologists, anaesthetists and vascular nurses.5 With an increasingly old, frail and co-morbid population presenting with AAA, it is recommended that a preoperative review from cardiologists and geriatricians is also included in any MDT discussion.6
MDT discussions add value due to the diverse clinical perspectives brought by different sub-speciality teams.7 Describing the potential sources of variation in AAA repair practice (OSR, EVAR and conservative management) between vascular centres, and how the MDT manages the patient pathway and supports clinical decision-making and patient involvement, is an essential step to identifying opportunities to improve patient care.5,8 This study aims to understand the reasons behind the variation in AAA repair practices across the UK in terms of MDT discussions and involvement of patients in decision-making. We also compare MDT implementation with best practice guidelines,5,6,8 and describe the information resources provided to patients to support decision-making.
Methods
Design
An observational cross-sectional organisational survey was undertaken with the clinical lead (or a consultant vascular surgeon nominated by the clinical lead) within participating NHS vascular centres.
Sampling and recruitment
The sampling frame included all 72 UK centres; we aimed to recruit a third (n=24). Rates of EVAR versus OSR and the proportion of patients reviewed by an MDT were obtained from the 2022 NVR annual report.2 Centres were categorised into high (>60%) versus low (<60%) EVAR utilisation rates, and low (<90%) versus high (>90%) rates of MDT review. The centres sampled were then stratified, with purposive recruitment aiming to achieve a balance across the four groups so participating centres were sufficiently diverse to reveal variations in centres’ organisation and delivery of AAA care. A member of the national (UK) Vascular and Endovascular Research Network (VERN: https://vascular-research.net/) contacted a local clinical lead in each centre and provided study information. Researcher (AW) organised interview times with participants, and verbal consent for study participation was confirmed prior to data collection.
Procedure
A study questionnaire (Item S1) was developed with an interdisciplinary and multiple stakeholder study team to capture details about:
• Centre and MDT composition and functional characteristics.
• Individual clinical decision-making practices within the context of the MDT.
• Integration of patient perspectives into MDT processes.
Clinicians received a copy of the survey in advance of the interview. Interviews were conducted using Microsoft teams or a telephone call. The researcher completed a paper copy of the questionnaire during the interview, capturing responses to closed questions and made brief notes on open-ended responses. Survey questions were read out verbatim to minimise interviewer bias. Video calls were recorded to ensure accurate capture of open-ended responses. A copy of the completed questionnaire was returned to the participant to check for accuracy. Participants were asked to provide copies of any written materials routinely provided to patients.
Data analysis
Data from the closed survey items were analysed using descriptive statistics (eg, proportions, medians and associated interquartile ranges (IQR)) and are presented in an aggregated format so individual centres cannot be identified. Responses to open-ended questions were transcribed verbatim and checked for accuracy before deleting the video recordings. Names and identifying characteristics were removed from data sets to ensure anonymity. Transcripts were coded and content analysis9 was undertaken. Interviews were coded iteratively, with preliminary codes revised in light of coding of subsequent transcripts and applied to all interviews. Consistent with a content analysis approach, no individual quotes were used in the presentation of findings, with data presented descriptively. A three-tiered framework was adopted to understand clinicians’ views on factors driving variation in AAA practice.10 Standards for reporting qualitative research were followed.11 The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist for observational, cross-sectional studies was followed.12
Research ethics and governance approval
The protocol was approved by the University of Leeds School of Medicine Research Ethics Committee (ref: MREC 21-059; 28/08/2022) and the UK Health Research Authority (ref: 22/HRA/5341; 24/01/23).
Patient and public involvement
A co-author and expert patient (LR) provided input to all stages of the project.
Results
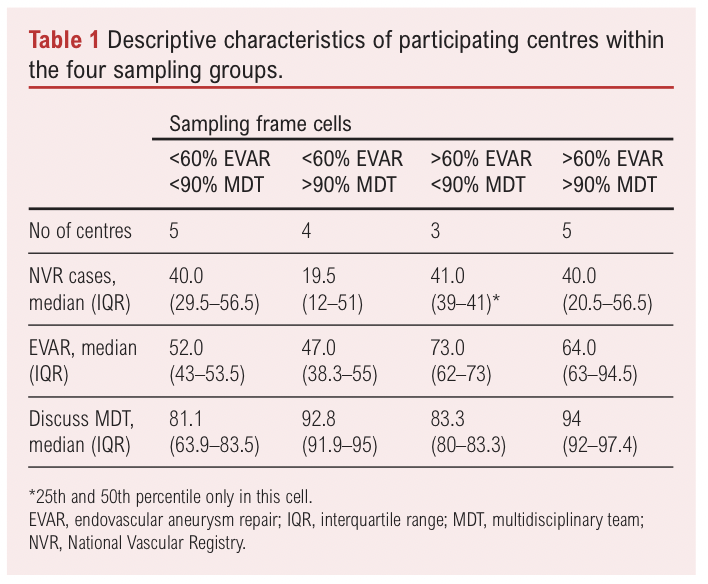
The VERN collaborator contacted consultant vascular surgeons at UK centres (n=72). One individual – either the clinical lead or vascular surgeon nominated to take part by the clinical lead – took part from each centre. Fifty of the 72 centres (69%) were contacted, at which point recruitment ceased due to time constraints. Seventeen (34%) of the 50 clinicians responded (24% of the total number of UK centres), divided roughly equally between the four groups (Table 1).
Composition of multidisciplinary team meetings
All 17 centres hold regularly timetabled MDT meetings at which patients with asymptomatic unrepaired AAA are discussed, and around half (n=8) convened additional MDT meetings to consider ‘complex’ patients (Table 2). The majority of centres reported a similar standardised procedure for referring patients to the meeting, supported by an MDT coordinator. Vascular surgeons and radiologists are invited and attend all regular vascular and complex aortic MDT meetings (Table 2). Almost all MDTs have clinical nurse specialists and administrative support in regular attendance. Geriatrician and anaesthetist participation was less common. No centres invited patients or carers to attend MDT meetings.
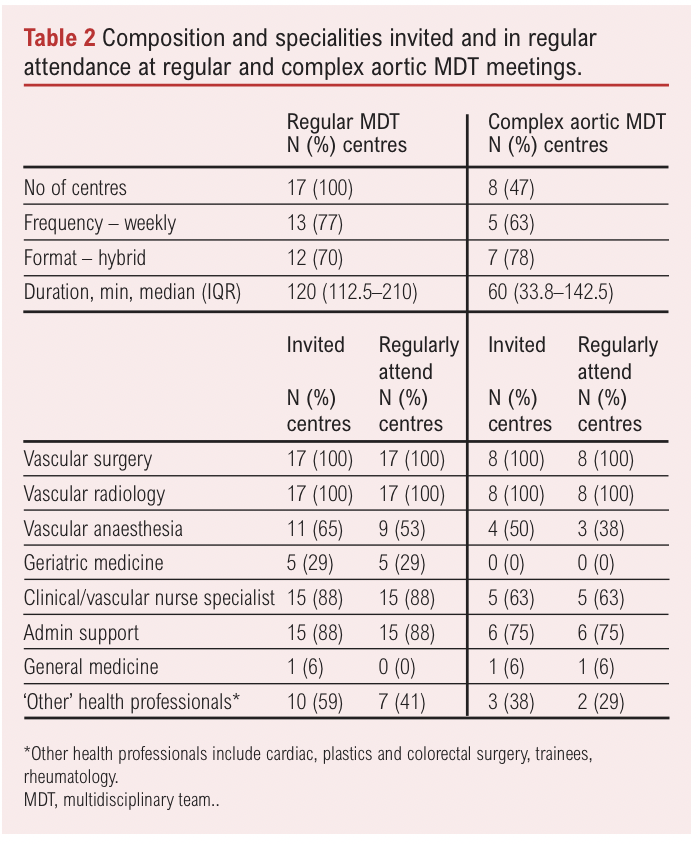
The majority of centres (n=14/17, 82%) have a quorate number of attendees for the regular vascular MDT and half reported a minimum quorum for attendance at complex aortic meetings (n=4/8, 50%). Centres varied in the number of specialties required to be present at a regular vascular MDT, reporting between 1–2 radiologists and 1–3 surgeons. Of the eight centres holding a complex MDT, four (50%) reported that at least one radiologist and 1–2 surgeons were the minimum requirement, three centres (38%) did not know the quorate number and one (12%) reported that an anaesthetist was required to attend.
Quality assurance measures
Two-thirds of centres had written criteria for patient referral (n=11, 65%). Around half of centres (n=8, 47%) had written terms of reference describing the minimum quorum and skill mix, and documentation, minuting and communication supporting the meetings (n=9, 53%). Similarly, around half reported a regular audit of clinical outcomes that was fed back to the MDT (n=7, 47%). Typically, centres reviewed unexpected outcomes in either the MDT (n=9, 53%) or another forum (n=15, 88%).
Clinicians’ beliefs about the role and function of the MDT
Clinicians (n=17) considered that the MDT is effective in achieving an evidence-based decision (median score 8 (IQR 6–8); scores range from 1 (not at all effective) to 10 (very effective). They reported being satisfied that MDTs include a range of specialties; decisions are based on ‘best available’ evidence; decisions are discussed and challenged, and processes are fair and transparent. A minority of respondents reported that MDTs are too time-pressured, especially for complex cases (n=3, 18%), too opinion-based (n=1, 6%) or have a tendency towards over-treatment (n=2, 12%).
Information used to support clinical decision-making
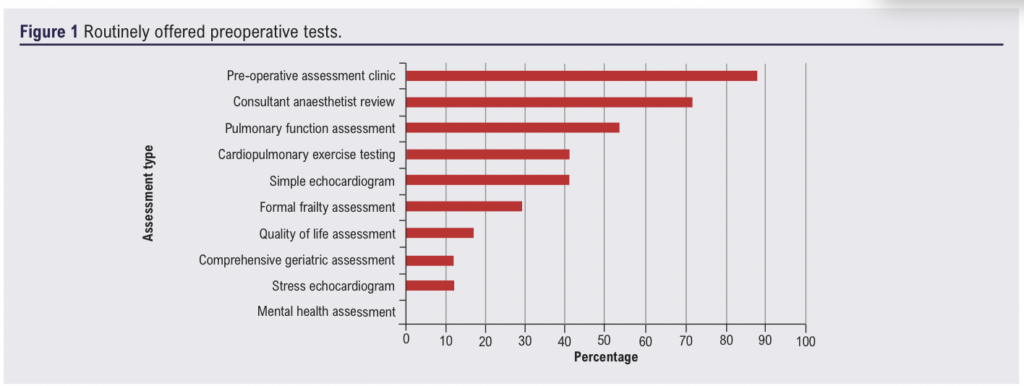
Routinely offered tests
Routinely offered tests are summarised in Figure 1. Most frequently, people are reviewed in a pre-operative assessment clinic (n=15, 88%) and by a consultant anaesthetist (n=12, 71%). Only two centres (12%) offer people with AAA a routine comprehensive geriatric assessment; five centres offered a ‘frailty’ review (n=3, 17%) or referral to a perioperative care for older people undergoing surgery (POPS) clinic (n=2, 12%).
Patient facing information
Patient facing information is provided from multiple sources including leaflets, introductory letters and web-based information (median 3 (IQR 2.5–3) sources per centre). Additional information included graphs of survival, risk information, NVR centre level data reports and the Carlisle risk prediction formula.
Patient information leaflets
Patient information leaflets are commonly used to supplement consultations. Fifteen centres provide written information to patients and 11 of these provided 16 leaflets to the investigators. Leaflets were produced in-house (n=10, 62%), by the Circulation Foundation (n=3, 19%), EIDO Healthcare (a private company producing health information resources to support informed consent) (n=2, 13%) and the Vascular Society (n=1, 6%). Five leaflets provided generic information about AAA and options for management and 11 were focused specifically on a particular repair type. None focused specifically on conservative management. We did not ascertain at which point in the patient pathway each leaflet was offered.
Leaflets presented risks as percentages, adjectives (high or low) or relative adjectives (higher, lower). Risks were overwhelmingly anchored to the risk of an event rather than the risk of no-event. No pictograms of risk were provided. No leaflet contextualised AAA risk within figures for overall (all-cause) medium- and long-term mortality risk. Uncertainties in the evidence base supporting repair decisions were not referenced in the majority of leaflets (14/16).
Six leaflets provided general health advice and management of cardiovascular risk. The effects of AAA diagnosis or repair on family, social and work life were described in 10/16 leaflets: the ability to drive, sexual function and exercise were the most common narratives. No leaflet provided advice on – or signposted to support for – the management of psychological aspects of an AAA diagnosis or end-of-life planning.
All procedure-specific leaflets provided detailed information on technique, likely inpatient experience, procedure risk, short- and long-term outcome and side effects. One leaflet included two vignettes of different decisions for repair. This leaflet advised thinking about personal priorities when making decisions. Other than this, no leaflets offered direction about how to make a repair decision or recommended discussion with friends, family, carers or professionals. All in-house leaflets signposted to telephone numbers for local clinical nurse specialists, vascular wards, and almost half to smoking cessation services (n=7, 44%) and the Circulation Foundation website (n=6, 38%). No leaflets signposted to a patient support network.
Describing risk and making decisions with patients
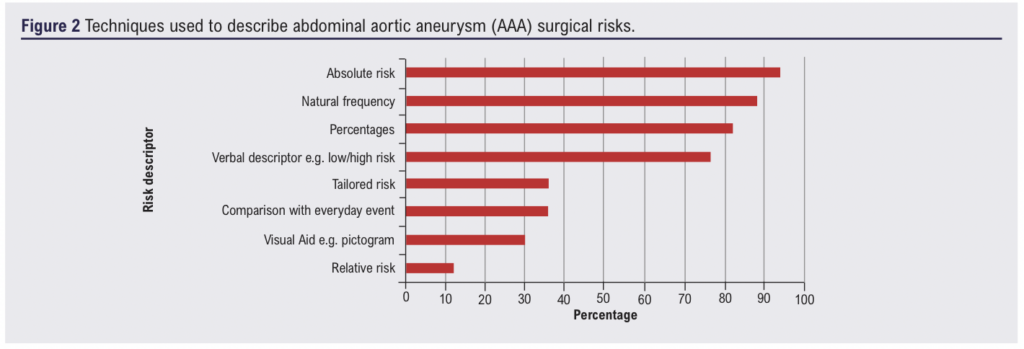
All participants reported using more than one method to discuss risk to support decision-making with patients (median 5 (IQR 3–6); Figure 2). Thirteen participants reported the annual risk of rupture for Caucasian males with a 5.5 cm AAA as between 1% and 5%; nine reported within this range for females. One participant reported the risk for males was between 6% and 10% and five reported within this range for females. A minority of participants used a verbal descriptor (n=3, 18%) or did not quote a figure (n=2, 12%). Risk of surgery was ‘usually’ (n=7, 41%) or ‘sometimes’ (n=7, 41%) contextualised within a patient’s all-cause mortality risk when they are judged to be frail or co-morbid. Few vascular surgeons used tools such as the Vascular-POSSUM and Carlisle Risk indicator13,14 to calculate all-cause mortality (n=3, 18%). Clinicians explained the decision options to their patients using a method (n=10, 59%) similar to the structure provided by the Choosing Wisely Patient Prompt – BRAN (benefits, risks, alternatives and doing nothing)15; two (12%) provided BRAN for their patients to use in consultations.
Including patient preference to support clinical decision-making
All participants reported ascertaining a patient’s preference for surgery and eight (50%) reported a formal process for doing so. The majority of centres reported making patients aware that they will be discussed in a MDT in their absence (n=14, 82%). Patient preference was elicited by a surgeon or anaesthetist and recorded in hospital notes, letters to the GP and MDT meeting minutes. Clinicians (n=15) rated the MDT as effective in achieving a patient-centred decision (median 8 (IQR 5–9); scores range from 1 (not at all effective) to 10 (very effective)). Participants stated that time was spent understanding patient preferences, and patient wishes were respected even when treatment preference differed from the MDT recommendation. Barriers to understanding patient preference were identified if the referring clinician did not attend the meeting. Three participants felt that some patients welcomed a steer from their clinician towards a particular decision. Two participants did not provide a rating as they did not consider patient preference relevant for inclusion in MDT discussion.
Management of people unsuitable for surgical repair
The majority of participants (n=14, 82%) reported that all patients considered for repair are discussed at MDT meetings, but older, co-morbid and/or frail patients and those whose aneurysm is discovered through incidental screening may not be discussed. The definition of those deemed not suitable for surgery varied. Some participants (n=2) disliked the use of the phrase ‘turn down’ to describe someone deemed unsuitable for repair or those choosing not to have an intervention. The decision to forgo repair was described as either patient-led, a joint discussion between patient and clinician, or based on clinical judgements about the patient’s best interests. Management of patients not undergoing repair included remaining on surveillance and offering repair at an increased AAA diameter, or removal from surveillance at the patient’s request. The decisions in this group are recorded variously, either by communicating the decision with a GP (n=2, 12%), in the hospital electronic record (n=5, 29%), MDT minutes (n=4, 24%), recorded on a spreadsheet (n=5, 29%) or a combination of these processes. Some reported no formal record keeping (n=2, 12%).
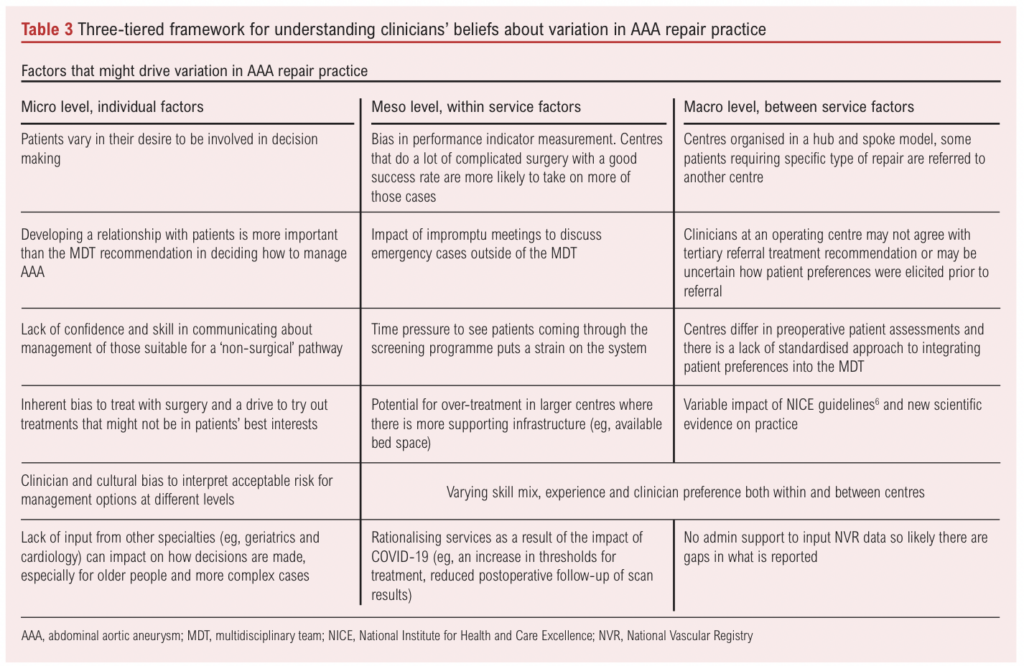
Clinician beliefs about what drives variation in practice
Clinicians provided views on what drives variation in practice. This is summarised in Table 3 using a three-tiered framework.10
Discussion
This survey, completed by consultant vascular surgeons from a quarter of UK vascular hospital centres, provides an overview of how MDT meetings are structured to support clinical decision-making about AAA repair surgery.
Understanding variation in practice composition of the MDT
Quality standards introduced in 2012 recommend that each patient with an AAA should be reviewed preoperatively by an MDT.5 All clinicians in participating centres reported regular MDT meetings, with around half also hosting MDTs dedicated to complex cases. There was considerable variation in the frequency, duration and skills mix of those invited and present at MDTs between centres, possibly accounted for by the size of centres. Internal governance procedures varied; not all MDT meetings are guided by standardised procedures and protocols or quality assurance mechanisms. This likely reflects the relative infancy of MDT meetings in this clinical space.15 Vascular surgeons and anaesthetists reflected that clinicians’ skills mix and differences in service infrastructure and referral patterns were likely to impact the pre-operative assessment and optimisation of patients undergoing elective AAA repair.16 A lack of diversity in specialities attending the MDT has the potential to bias decision-making, as each speciality has its own goals and protocols, and without their contribution the overall clinical reasoning of a team may be impacted. Barriers to participation of all specialties were outside the scope of this study. However, previous work has described issues relating to both funding and availability of specialties such as geriatric medicine to attend surgical MDTs.17 Enablers may include appropriate job planning or facilitative approaches such as virtual MDT meetings.
Information to support or bias shared decision-making with patients
NICE guidelines recommend that people with AAA are provided with information about their options for repair or conservative management, including risk figures and information about uncertainties from the evidence.6 Most commonly, clinicians report presenting treatment options in an outpatient consultation by describing an approach consistent with ‘benefits, risks, alternatives and doing nothing’ (BRAN). Presenting AAA repair as a choice between options is more likely to support patients to make trade-offs between management plans. However, written information provided about AAA repair is not balanced, focusing on preparing for surgery or making decisions between types of surgical procedures. Conservative management was not described actively. Patients are unlikely to be able to weigh up the pros and cons of ‘doing nothing’ unless it is framed using the same attributes as repair options.
Figures describing the annual risk of rupture were variable. Annual risk of rupture for men with an AAA of 5.0–5.4 cm is estimated at 0.4%.6 While the contemporary annual rupture risk for a 5.5 cm AAA is unknown, participants quoted a figure ranging between 1% and 10% for both men and women. Using a patient decision aid to present accurate and balanced treatment information of all options may support people to make AAA repair decisions aligned with their preferences.18 Adopting a user-centred design approach to their development may help ensure that information supports people with lower health literacy.19 Some centres reported the use of risk assessment tools, despite this being contraindicated in NICE guidance.6 Enhancing clinicians’ skills to share individualised risk information of options and ameliorate unconscious bias may help both parties to agree and implement a treatment plan.20,21 It would be reasonable to suggest that the effective implementation of the NHSE Decision Support Tool rests on a reasonable degree of consistency of practice across centres.
Adopting a non-surgical approach to managing AAA
There was a lack of consensus about the definition of someone not deemed suitable for (or declining) repair, how this is recorded, and the subsequent management pathway in lieu of repair. Participants reported organisational and clinical factors that may lead to overtreatment. This is likely compounded by limited MDT input from geriatricians and patient information leaflets presenting narrow information about repair techniques. Framing treatment information as choices, with explicit options, and presenting this information in parallel in an option-by-attribute format is less likely to bias peoples’ preferences.22 Preliminary data from the use of AAA decision support tools suggest that they may lead to people choosing less invasive, non-surgical options.23 Creating a ‘non-surgical’ conservative management pathway within centres for those deemed unsuitable for repair would benefit the older, more frail, co-morbid population diagnosed with AAA.
Study advantages and limitations
Recruitment at centres was limited to those where the VERN collaborator was able to identify a named contact. The recruitment target was not met and interviews were difficult to secure, perhaps in part due to a stipulation in governance approval that relied on consultants taking part at a time that did not have an impact on their clinical duties. Adopting an interview approach to collecting survey data meant that answers supporting numerical rating scores could be fully explored.
However, some limitations are integral to the survey methods. The survey was aimed at clinical leads; in some centres the vascular lead nominated a member of their team to participate on their behalf. Their views may not be representative of all surgeons and the wider team working within each centre. Some of the items (eg, risk figure estimates and the use of patient materials to support decision-making) may be more susceptible to clinicians reporting on their own practice rather than wider delivery within their centre. Recording of patients discussed at a MDT is not a mandatory field in the NVR dataset. As such, there may be an underestimation of MDT activity due to absence of documentation. Data were not collected on the number of patients discussed at the MDT at each centre. This variation by centre may have an impact on the length of time taken to discuss each patient and the quality of the discussion, as mentioned by some clinicians in reference to more complex cases.
Conclusions
Although MDTs were universally adopted, the skills mix, remit and delivery varied between centres and there was considerable variation in how treatment options, risk information and uncertainty is presented to patients to support a shared decision-making approach. These factors may in part explain why there is variation in AAA repair practice nationally.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2024;3(4):231-238
Publication date:
August 26, 2024
Author Affiliations:
1. Senior Health Services Researcher, Leeds Teaching Hospitals NHS Trust, Leeds, UK
2. Professor of Medical Decision Making, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK
3. Consultant in Geriatric Medicine, Guy’s & St Thomas’ NHS Foundation Trust, London, UK
4. Consultant Vascular Radiologist, Leeds Teaching Hospitals NHS Trust, Leeds, UK
5. Associate Professor of Anaesthesia University of Leeds & Consultant Vascular Anaesthetist, Leeds Teaching Hospitals NHS Trust, Leeds, UK
6. Professor of Vascular Surgery, Department of Cardiovascular Science, University of Leicester and National Institute for Health and Care Research Leicester Biomedical Research Centre, Leicester, UK
7. Public Health Registrar, Nuffield Department of Population Health, Oxford University, Oxford, UK
8. Patient and Public Involvement Representative
9. Professor of Health Services Research, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK
Corresponding author:
Suzanne H Richards
Professor of Health Services Research, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK
Email: s.h.richards@ leeds.ac.uk











