CASE REPORT
Distal venous arterialisation for ‘no-option’ chronic limb-threatening ischaemia
Beck KJ,1 Howard DPJ2,3
Abstract
Chronic limb-threatening ischaemia (CLTI), defined as significant peripheral arterial disease causing ischaemic rest pain and/or tissue loss, is associated with a high amputation and mortality rate. Avoiding amputation in CLTI is crucial and restoration of blood flow is usually achieved using endovascular or open surgical revascularisation. However, significant occlusion of the distal limb vasculature may result in ‘no option’ CLTI, where there are no available target vessels for angioplasty or bypass. An emerging procedure to treat patients with ‘no option’ CLTI is distal venous arterialisation (DVA). This involves using the distal venous system as a conduit for arterial blood to revascularise the lower limb. This report describes a patient presenting with ‘no option’ CLTI who underwent limb-saving treatment with DVA. The case highlights how worsening clinical outcomes may occur despite technical success of DVA. It also emphasises complications of the procedure. Finally, the evidence base surrounding DVA for CLTI is examined.
Introduction
Peripheral arterial disease (PAD), which causes narrowing or occlusion of the arteries and reduced blood flow to the affected limb, affects 13% of the western population over 50 years old.1 As PAD progresses and becomes severe, it can result in critical limb-threatening ischaemia (CLTI). This is characterised by ischaemic rest pain and/or tissue loss in the form of non-healing ulcer or gangrene.2
It is estimated that at 1 year following presentation with CLTI, 25% of patients will die and 30% will undergo amputation.3 The mean 5-year care cost for patients with CLTI is estimated at 46,281€, and every above-knee amputation increases care costs by 25,692€. Therefore, the long-term care costs to the National Health Service (NHS) following CLTI are considerable.4
Depending on the severity at presentation, initial management of CLTI involves pain and pressure relief, antiplatelet therapy, antimicrobial therapy and wound care.5 Revascularisation through endovascular or open approach is often urgently pursued, aiming to restore blood flow to the affected limb, improve wound healing, preserve limb function and mobility, and avoid amputation. Unfortunately, some patients exhibit severe forms of PAD, extending below the ankle with significant occlusion of the distal limb arteries. This could result in a phenomenon called ‘no option’ CLTI or ‘desert foot’, where there is no suitable vessel for endovascular angioplasty, stenting or even open bypass surgery.6-8 It is estimated that 14–20% of patients fall into this category of ‘no option’ CLTI, and their prognosis is usually poor.9
An exciting potential option used as a last resort to restore blood flow to the foot in patients with ‘no option’ CLTI is distal venous arterialisation (DVA). It involves creating a connection between the tibial artery and vein, diverting arterial blood into the venous system to perfuse the distal lower limb.7,10,11 Halstead and Vaughan first proposed using the venous bed as a conduit to perfuse the peripheries in 1912.10 Open surgery was then first used in the 1970s, with case reports describing anastomosis of an arterialised great saphenous vein to the dorsal venous arch of the foot.7 Since then, there has been a move to percutaneous approaches for DVA. Here we present a case of a patient who presented with ‘no option’ CLTI and successfully underwent limb-saving treatment with percutaneous DVA.
Case history
A 54-year-old man had an 8-month history of increasing pain on the right foot, redness of the medial aspect of the right calf with gangrene of the right second toe. He was a previous smoker (30-pack year history) and has type 2 diabetes mellitus with diabetic retinopathy, hypertension, asthma and thalassaemia. His current medication included metformin 500 mg, atorvastatin, ramipril as well as a Clenil Modulite inhaler, and his HbA1c was 5.2%. He had a long-standing history of PAD with previous right third toe amputation and multiple previous attempted angioplasties, including both antegrade femoral access and retrograde pedal access.
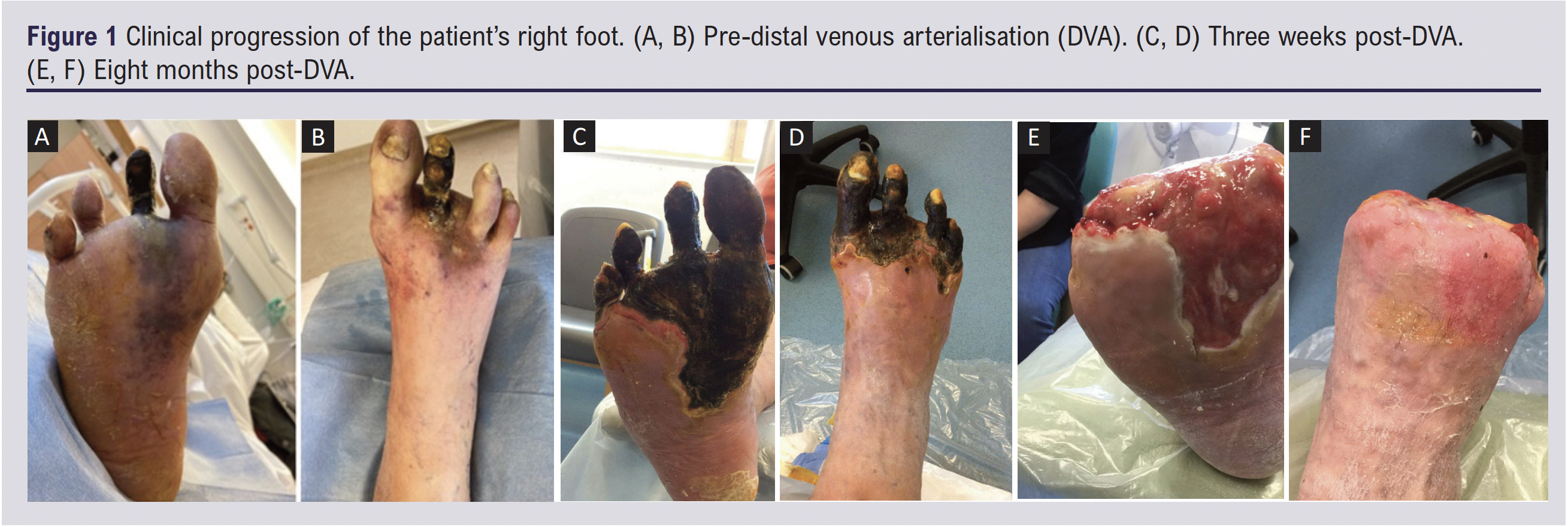
On examination, the right leg was cool peripherally, with pallor of the digits, and both erythema and mild pitting oedema of the forefoot and lower calf. The right second toe was necrotic and black, with dusky discolouration on the dorsal aspect of the foot (Figure 1A and B). On the right leg, the femoral and popliteal pulses were palpable. Examination of the right dorsalis pedal (DP) and posterior tibial (PT) arteries with a hand-held doppler ultrasound revealed damped monophasic waveforms. Toe pressure measurement was 18 mmHg on the right and 43 mmHg on the left. Overall, his presentation was consistent with Rutherford category 5 CLTI with a Wound, Ischaemia and foot Infection (WIfI) stage 4.
An arterial ultrasound duplex scan of the right leg demonstrated calcification and occlusion of the anterior tibial artery (ATA) and peroneal artery (PA), as well as distal stenoses in the PT artery. Initial management included continuation of his antiplatelet, antihypertensive, antidiabetic and statin therapy, with addition of antibiotics (intravenous co-amoxiclav and oral metronidazole) for right foot infection. A right leg angiogram revealed a patent superficial femoral artery and popliteal artery, but the distal ATA was heavily calcified and occluded (Figure 2A). The occluded ATA was treated with angioplasty using a 3 mm balloon. Occlusions were also found in PT and DP arteries, but these could not be traversed. On arterial ultrasound duplex, there was no distal artery that was a suitable target for open bypass surgery. Arteriovenous imaging ascertained a Global Limb Anatomic Staging System (GLASS) stage III. Because of the failure of endovascular treatment and impossibility of open bypass, the patient was considered for DVA.
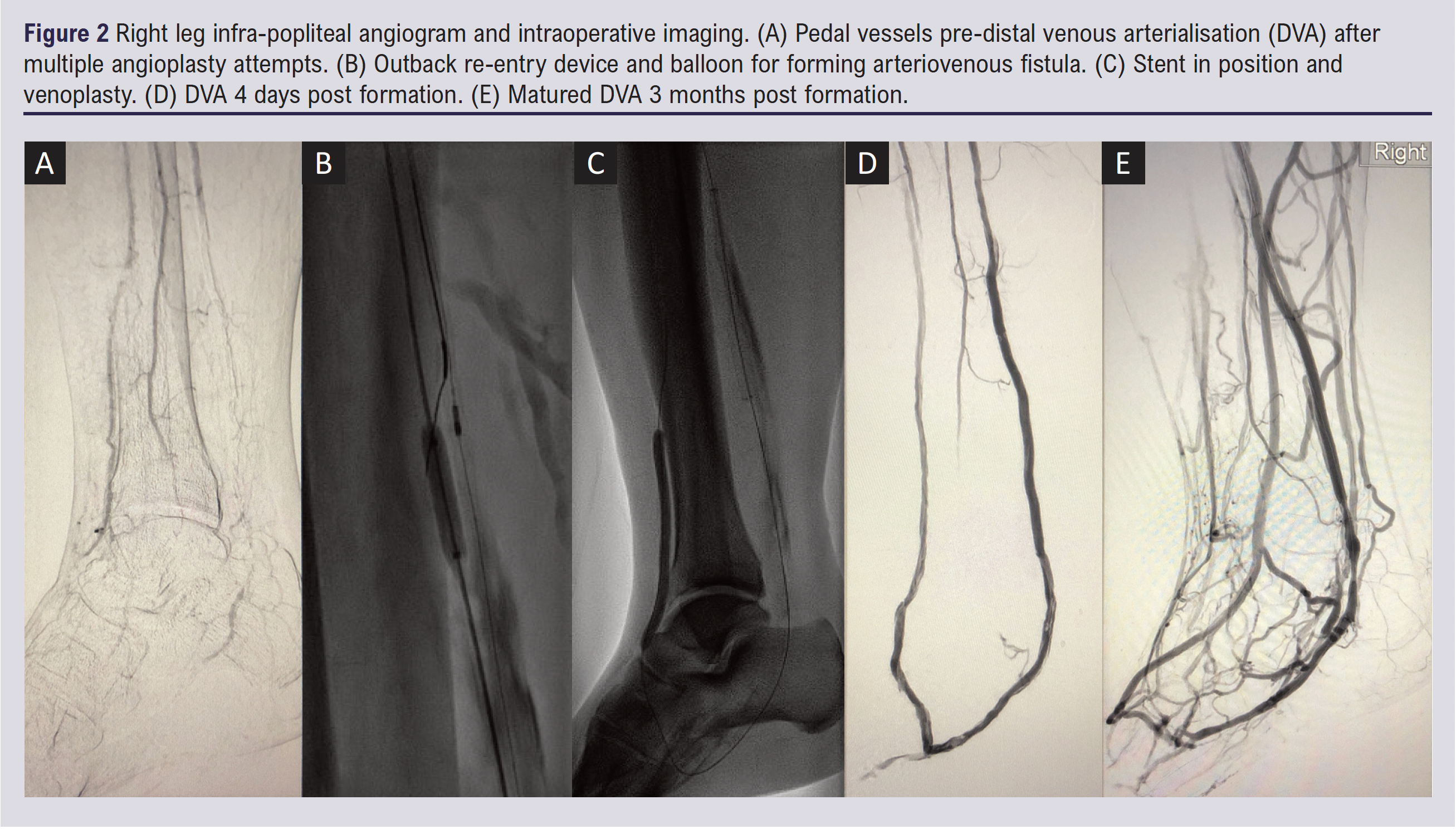
The DVA procedure was performed at the major vascular centre in our network. This procedure was pre-approved by the Trust Technologies Advisory Group Committee and was performed under anaesthetic by an experienced vascular and endovascular surgeon and interventional radiologist. Patients are carefully consented and are made aware that the procedure is novel and long-term outcomes are currently unknown. The DVA procedure was carried out as follows: the PT artery and posterior tibial vein (PTV) were cannulated. The proximal PT artery was dilated with angioplasty and an outback re-entry device was used to create a fistula from the proximal artery into the vein (Figure 2B). Stents were placed across the arteriovenous fistula (Papyrus Biotronik 4×26 mm, Viabahn 5×100 mm). The PTV was then aggressively venoplastied to optimise blood to flow into the foot (Figure 2C). All significant valves in the PTV were rendered incompetent with balloon venoplasty (standard 5×100 mm, Angiosculpt 6×100 mm balloons). During the operation thrombus formed in the stent but was removed via mechanical thrombectomy.
Postoperatively, the patient initially had no complaints of pain. On examination, the right foot was pink and warm with a strong palpable thrill present in the venous arch of the foot. Three days post-DVA, right leg arterial duplex demonstrated the PT artery to PTV fistula was patent, indicating a technically successful procedure. However, 4 days post-DVA increasing pain and erythema were noted in the patient’s right leg. This resulted in further angioplasty of the fistula outflow vein with a 5 mm balloon, improving the flow of the distal arch veins seen on angiography (Figure 2D). Following this, the pain improved and the patient was discharged to his home 3 days later.
At 3-week follow-up a well demarcated spreading necrosis on the plantar surface of the right foot was observed (Figure 1C, D). The calf of the patient was erythematous and he reported tiredness. At this point the foot was still deemed at risk of ischaemia and thus below-knee amputation was still an option.
The patient was regularly followed up in a weekly multidisciplinary diabetic foot clinic, ensuring regular debridement of necrotic tissue and regular review of antibiotic therapy. A ‘watch and wait’ approach was adopted and, eventually, no further operative procedures were needed. Over the next months the remaining toes gradually autoamputated leaving behind healthy granulation tissue (Figure 1E, F). The right PT venous pulse remained palpable with good doppler signal, and follow-up right leg arterial duplex demonstrated continued patency of the arteriovenous fistula. He reported feeling well in himself and denied significant pain in the right foot. Ultimately, the vitality of the mid and hindfoot had been preserved and below-knee amputation had successfully been avoided. This highlights that, in spite of technical success, DVA may not initially improve clinical outcomes with eventual worsening of gangrene before improvement.
In terms of functional improvement, the patient went from requiring a wheelchair for most activities to having completely independent mobility and being able to drive in a car and return to work.
The patient provided written informed consent for his case to be written up as a case study.
Discussion
The crux of a successful DVA is dedication to ensuring that excellent flow is achieved around the venous foot arch. This requires endovascular disruption of all venous valves and potentially embolisation of any large collateral veins that would divert blood away from the distal forefoot. This patient demonstrates that, if this occurs successfully, DVA can be used to avoid amputation, improve wound healing and improve quality of life in patients with ‘no option’ CLTI. Although this is a new and novel technique, initial subclinical deterioration in tissue loss has been recognised and we can confirm this in our case series so far. This is likely to be due to the sudden haemodynamic changes caused by the formation of the DVA. Immediately after formation the arterialised vein can potentially ‘steal’ blood flow from the distal forefoot and digits as it is a high-flow fistula without any maturation. As the vein arterialises and matures, the microcirculation to the foot starts to be pressurised by this new vessel with oxygenated blood being driven in reverse through the venules and arterioles. The foot often swells and looks erythematous for 3–6 weeks post intervention and then this process subsides. Nonetheless, careful use of regular multidisciplinary foot clinic review and staged debridement ensures wound healing eventually occurs.
Outcomes of systematic reviews and retrospective studies assessing DVA for CLTI
Reports of DVA as a treatment option for ‘no option’ CLTI have only emerged in the last two decades, mostly in the form of case reports and retrospective cohort studies. Summarising the observations of 16 retrospective cohort studies looking at a total of 768 patients undergoing DVA for CLTI, Schreve et al reported a mean 1-year limb salvage rate of 75% (95% CI 70% to 80%).11 Limb salvage is defined as the percentage of subjects free from above ankle amputation of the affected limb. Other outcomes included 30-day hospital mortality, which ranged from 0% to 10%, and overall survival, ranging from 54% to 100%. The post-surgery patency of venous arterialisations, usually measured using duplex ultrasound in the days following surgery, has been reported as 66–72%.11 More recently, the largest retrospective cohort study to date assessed 32 patients with Rutherford category 5 or 6 ‘no option’ CLTI receiving DVA with a Limflow device.12 It reported a 97% technical success rate, with limb salvage rates 86.8% at 6 months, 79.8% at 12 months and 79.8% at 24 months. Notably, there was also a statistically significant increase in transcutaneous oxygen pressure (TcPO2) from 14.5±12.7 mm Hg before surgery to 56.1±11.9 mm Hg 2 years after DVA. TcPO2 measurements demonstrate perfusion status in the microvasculature of the foot and are a direct predictor of wound healing.13,14
Complications of DVA identified in retrospective studies include postoperative oedema, thrombosis (as was the case in this patient’s surgery), major cardiac events, bleeding and infection.12,15 Perhaps the most significant flaw of DVA is the loss of DVA primary patency. Lu et al reported that, in 144 patients undergoing DVA, after 1 year DVA patency was only 46% (95% CI 39% to 53%).15 Likewise, others report a reintervention rate due to loss of primary patency of 65%.12 Furthermore, whilst major amputation occurrence is reduced through DVA intervention, notably among those going on to require amputation, angiography demonstrates no occlusion of the DVA in 75% of cases.12 Duplex imaging of the DVA every 6–8 weeks for the first 6 months post procedure is important due to the risk of developing venous valve stenoses or neointimal hyperplasia at the distal stent transition which may require expedited reintervention to maintain DVA patency.
Limitations of retrospective cohort studies assessing DVA for CLTI
Since only retrospective observational cohort studies are currently available to assess DVA for CLTI, publication bias is a major issue in the field as positive results following DVA are more likely to be published. Given how rarely DVA is performed, the numbers of participants reported in individual studies are very low. There is a lack of randomised controlled trials comparing DVA with other limb-saving procedures for CLTI (endovascular and bypass surgery) due to the ‘last resort’ nature of the procedure. Designing a randomised controlled trial to ascertain the success of DVA is complicated; given its novelty and associated learning curve, it is difficult to accurately compare it with established procedures for CLTI – namely, surgical bypass and endovascular intervention. Other experimental non-surgical treatment options for ‘no option’ CLTI patients exist – for example, spinal cord stimulation, lumbar sympathectomy, pharmacotherapy (prostanoids, vasoactive drugs) or stem cell therapy.5 Future studies could aim to compare DVA with these approaches. Alternatively, one could compare patients having DVA with those who go on to have amputation without consideration for DVA using outcomes such as survival, physical function and quality of life.
In addition, there is a lack of standardisation among cohort studies in terms of outcome measures: many studies fail to report on one or more of patient reported outcomes such as subjective rest pain resolution or improvement in carrying out activities of daily living, precise haemodynamic outcomes with measurements such as ankle brachial pressure index (ABPI) or TcPO2, or anatomical reporting in terms of patency seen on duplex or angiography. The outcome of ‘above ankle amputation-free survival’ does not take into account varying degrees of amputation above the ankle, which could vary in impact on the patient’s quality of life. Given in our case there was often a discrepancy between patient reports of pain, the technical success of DVA formation shown on angiography and the assessment level of necrosis visible on the foot, we suggest a detailed assessment of outcomes following DVA should be undertaken to include subjective (patient reported pain and other symptoms), haemodynamic and anatomical outcomes. A validated recommended core outcome set for CLTI studies would be invaluable.
Furthermore, patients who undergo DVA for CLTI are heterogeneous. It is difficult to undertake a nuanced subgroup analysis factoring in, for example, the extent of diabetes management or WIfI and GLASS classification, all of which influence amputation-free survival and overall mortality, given the small number of patients undergoing the procedure. As DVA becomes more routine, more evidence will emerge to facilitate a refined understanding of exactly which categories of CLTI DVA is most effective for.
Future directions
The PROMISE II trial (ClinicalTrials.gov identifier NCT03970538) is currently assessing the safety and efficacy of DVA using the LimFlow device for ‘no option’ CLTI in 120 participants based in the USA.16 Outcomes include major amputation-free survival, primary and secondary patency and wound healing. Of note, the incidence of contrast-induced nephropathy will also be assessed, which is missing from most reports.11,12,15 The technique used in our centre is similar to the LimFlow device, but potentially cost saving as it is performed with standard endovascular access, wires and devices.
In order to effectively provide a robust evidence base for DVA, ideally PROMISE II will address several limitations of the existing literature. Firstly, it should report multiple outcomes such as subjective patient-reported measures, functional assessment of patient mobility as well as precise haemodynamic measures such as ABPI and TcPO2. Furthermore, patients in the trial should be followed up with regular arteriovenous imaging to determine DVA patency and determine which factors could be linked to re-occlusion and loss of patency, as this appears to be the main drawback of the procedure.17,18 Finally, trials could account for subject heterogeneity by stratifying patients into different subgroups. Assessing these subgroups separately will enable the creation of a model that predicts for whom DVA would be most effective and appropriate. The PREVENT III risk score has already been established for estimating amputation-free survival in patients with CLTI undergoing infrainguinal bypass – a similar risk score could be established here.17
We propose that, in conjunction with PROMISE II, centres performing DVA should carefully monitor the incidence of intraoperative complications, follow up long-term patency and link these to functional assessments of patient mobility and quality of life (eg, using SF-36 questionnaires). This will facilitate a holistic assessment of the role of DVA in improving the lives of a complex group of patients with multiple co-morbidities.
Conclusion
For patients with ‘no option’ CLTI, amputation has traditionally been likely. Ultimately, this patient demonstrates that DVA is a promising option for patients with ‘no option’ CLTI, avoiding amputation, improving wound healing and patient mobility. Notably, this case highlights two important complications of DVA: thrombus formation and re-intervention to maintain patency. These are key areas that need to be addressed in this continuously developing area of vascular surgery. Furthermore, initial temporary extension of forefoot necrosis is often observed after successful DVA formation. With off-loading, regular foot clinic review and staged debridement we have found this stabilises and excellent granulation tissue formation and wound healing eventually occurs. Our case suggests that, in patients in whom a DVA remains patent for at least 8–12 weeks, healing and avoidance of major amputation is possible.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2022;1(3):100-104
Publication date:
March 29, 2022











