CASE REPORT
Explantation of infected kissing iliac stents complicated by acute discitis with aortoiliac reconstruction: a case report
Abdelmalak M,1 Waseem F,2 Wallace S,1 Karouki M,1 Torella F,1,3,4 Sabbagh C1
Abstract
Introduction: Endovascular therapy for aortoiliac occlusive disease (AIOD) has the benefits of being minimally invasive with reliable patency outcomes. The VIABAHN VBX Balloon Expandable Endoprosthesis is widely used for iliac interventions; however, infection of such devices is exceptionally rare and its clinical course poorly characterised.
Case presentation: A 55-year-old man with a history of mucosa-associated lymphoid tissue lymphoma presented with lifestyle-limiting claudication due to left common iliac artery occlusion. Following multidisciplinary team (MDT) review, he underwent successful percutaneous kissing iliac stenting using VBX endoprostheses. One week later he re-presented with new onset back pain and left leg swelling. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) demonstrated focal tracer uptake around the left iliac stent with maximum standardised uptake value (SUVmax) 10.6, and blood cultures grew Staphylococcus epidermidis. Despite intravenous antibiotics, the pain worsened and repeat PET/CT revealed progression (SUVmax 15.6) and L4 vertebral erosion consistent with discitis and osteomyelitis.
Management and outcome: Following MDT consensus, both stents were explanted and the aortoiliac segment reconstructed in situ with a custom bifurcated graft fashioned from bovine pericardium. Cultures confirmed S. epidermidis. Guided by susceptibility testing and European Society for Vascular Surgery recommendations, ceftriaxone (2 g IV daily) and rifampicin (600 mg orally daily) were administered for 10 weeks. Serial MRI demonstrated resolution of the 26 × 19 mm prevertebral abscess and regression of inflammatory changes. At six-month follow-up the patient remained infection-free with restored mobility, reporting only retrograde ejaculation – recognised consequence of open aortic surgery.
Conclusion: This appears to be the first documented infection of a VBX endoprosthesis complicated by early vertebral discitis. Rapid diagnosis through multimodal imaging and coordinated surgical–infectious disease management enabled cure. Clinicians should maintain vigilance for this rare but severe complication, particularly in immunocompromised patients.
Introduction
Recent advances in endovascular devices, coupled with increased operator experience, have expanded the use of endovascular therapy for treating extensive aortoiliac occlusive disease (AIOD). These procedures are now frequently employed as first-line treatment, largely due to the perception that surgical options remain viable if endovascular approaches fail.1 Nevertheless, several important questions persist regarding this evolving treatment paradigm. While the theoretical risk of infection associated with vascular endoprosthesis is recognised, the occurrence of such events with iliac stents remains exceedingly rare and data on their incidence and outcomes are limited.
Case report
We report a case of a 55-year-old man who presented with progressive life disabling claudication involving the buttocks and calves not responsive to best medical therapy and supervised exercise. His past medical history was notable for chronic obstructive pulmonary disease, peripheral vascular disease and stage IVSE terminal ileum mucosa-associated lymphoid tissue (MALT) lymphoma, previously treated with six cycles of rituximab, cyclophosphamide, vincristine sulfate and prednisone, completed one year prior to presentation.
Computed tomography angiography (CTA) showed a total occlusion of the left common iliac artery (Figure 1). The case was discussed in a peripheral vascular disease multidisciplinary team (MDT) meeting and the patient elected to proceed with the endovascular option. Percutaneous iliac angioplasty and stenting were performed using a standard technique, with intravascular arterial pressures measured via an arterial line pressure catheter. Initial stent deployment was achieved using VBX Balloon Expandable Endoprostheses (W. L. Gore & Associates, Flagstaff, Arizona, USA): an 8 × 79 mm stent on the left and an 8 × 59 mm stent on the right. The right stent was subsequently post-dilated to 10 mm within the right common iliac artery (CIA).
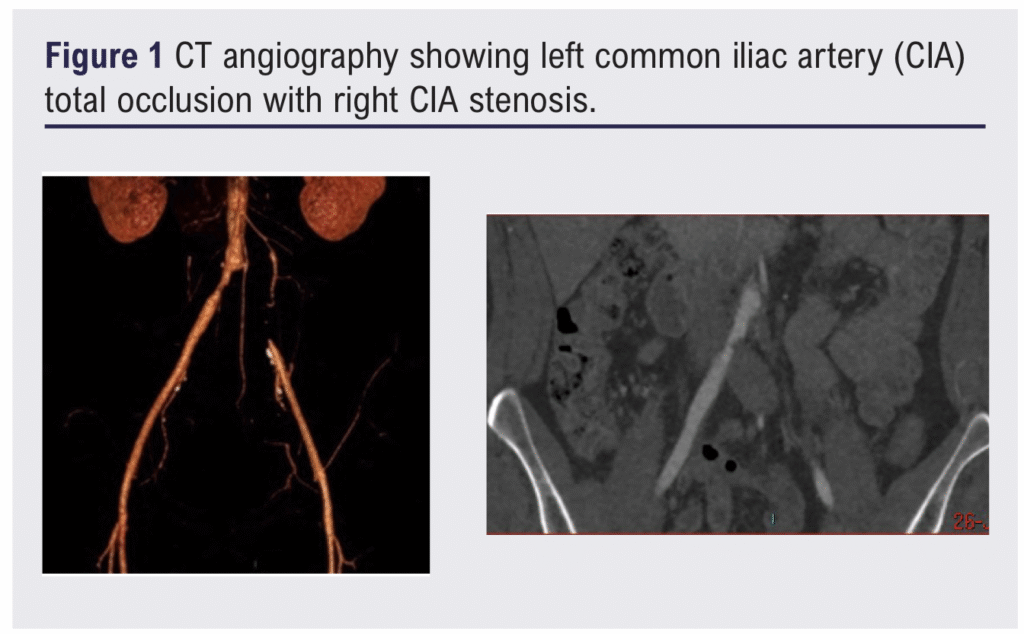
Completion angiography showed a filling defect at the proximal aspect of the left stent, likely due to a fibrin cap at the apex of the chronic occlusion. To optimise flow, the iliac stents were extended bilaterally using additional VBX stents: 8 × 39 mm on the left and 9 × 39 mm on the right, inflated to 7 atmospheric pressure (ATM) to limit expansion to 8 mm. Final angiography showed satisfactory flow through both stents with preserved three-vessel runoff bilaterally (Figure 2). Post-procedural intravascular pressures were recorded as follows: left external iliac artery (EIA) 176/80 mmHg and right EIA 170/75 mmHg. The procedure went uneventfully, and the patient was discharged on dual antiplatelet therapy.
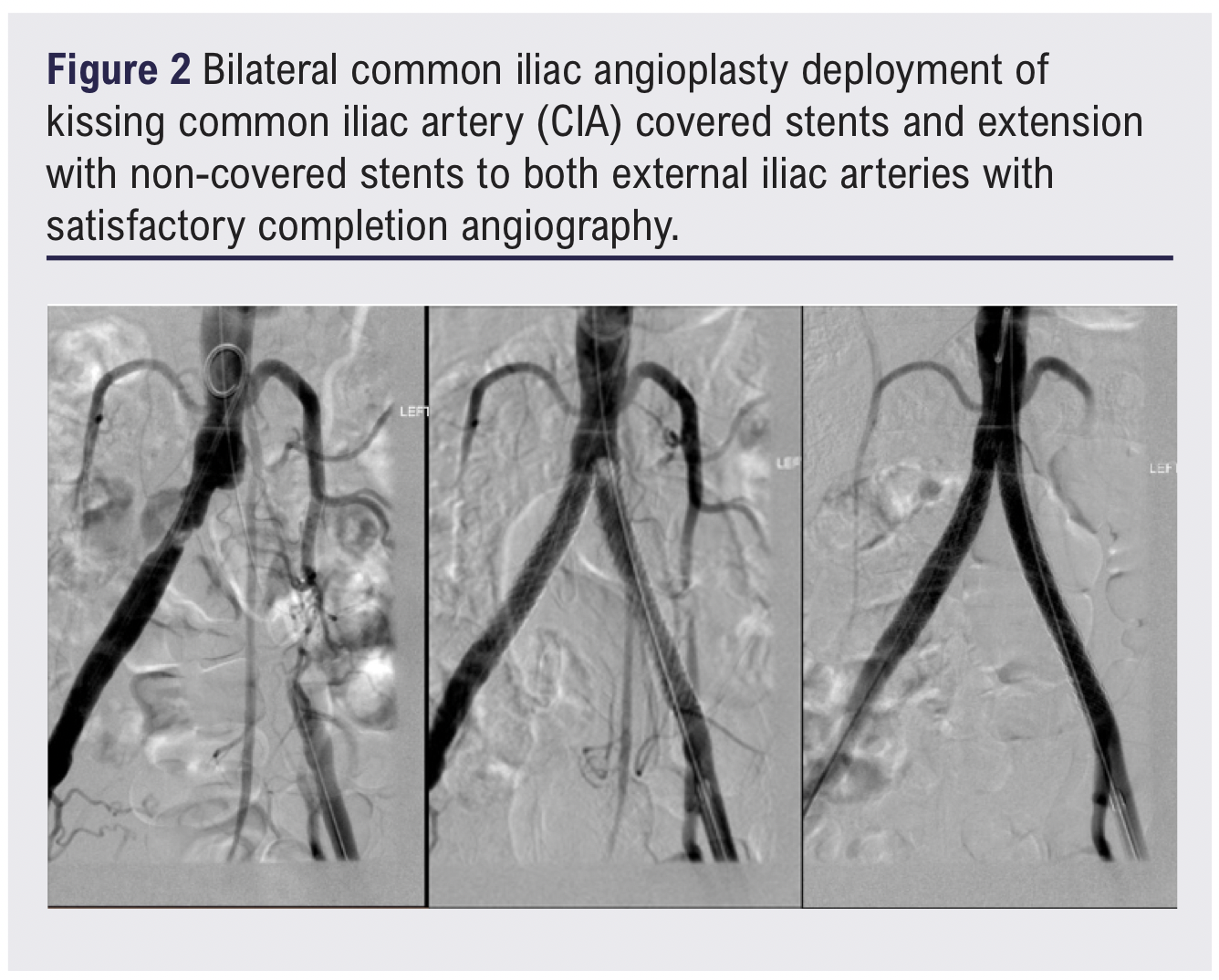
One week following the procedure the patient presented to the emergency department with mild left leg swelling and progressive unfamiliar back pain. CTA and duplex ultrasound confirmed stent patency and did not reveal any acute complications to account for the symptoms. However, fat stranding was noted around the left iliac stent and was interpreted as a potential early sign of peri-stent infection (Figure 3). A full peri-iliac stent infection work-up was initiated, including updated inflammatory markers, blood cultures, repeat CTA and a positron emission tomography-computed tomography (PET-CT) scan, which revealed occlusion of the left iliac stent.
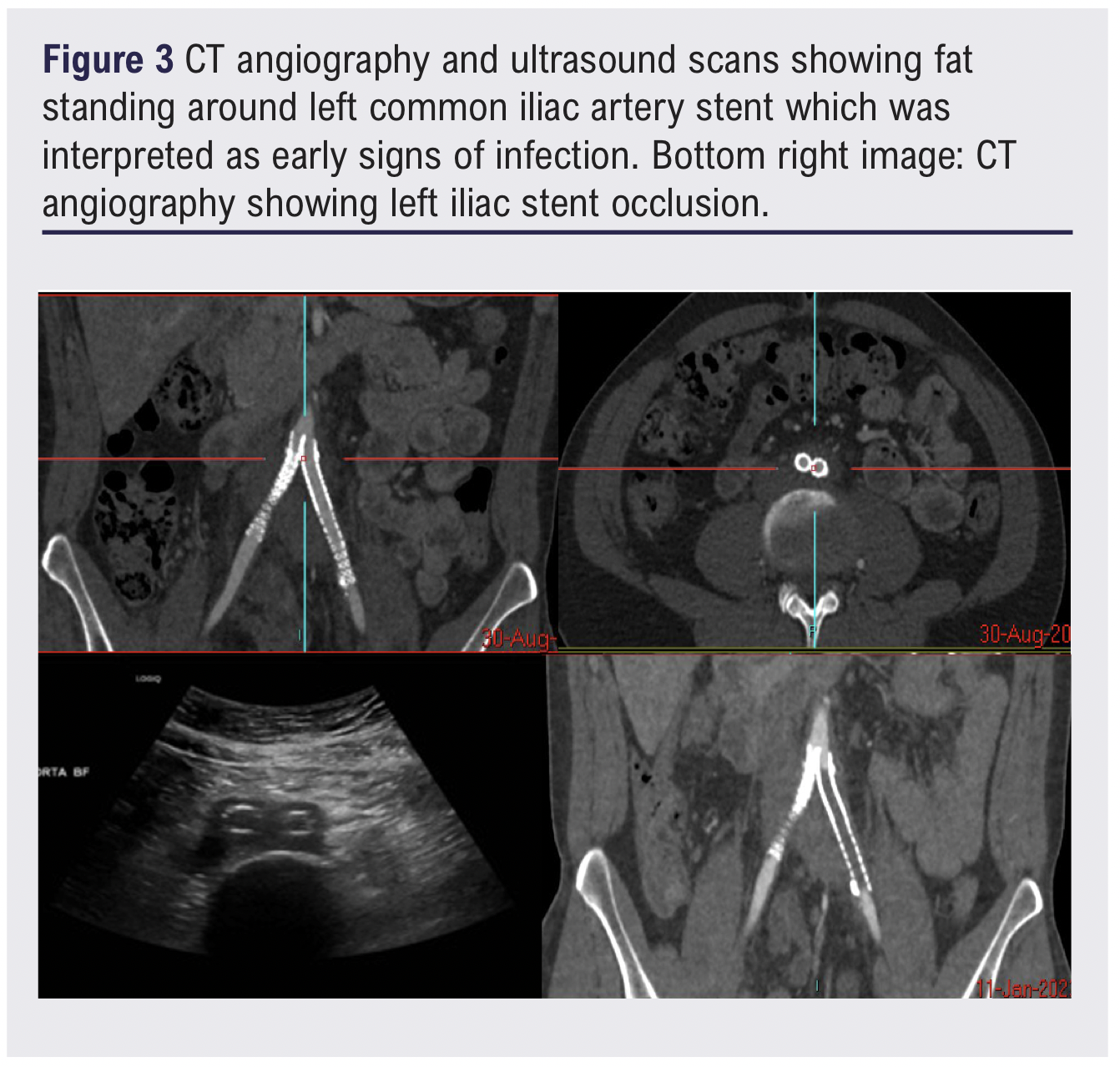
The case was re-evaluated at the interventional radiology and vascular MDT, who initially agreed on a conservative management strategy. A repeat PET-CT scan was recommended if symptoms persisted. Blood cultures subsequently returned positive for Staphylococcus epidermidis, and the PET-CT scan demonstrated increased 18F-fluorodeoxyglucose (FDG) uptake around the left common iliac stent with a maximum standardised uptake value (SUVmax) of 10.6. Based on microbiology advice, intravenous antibiotic therapy was initiated.
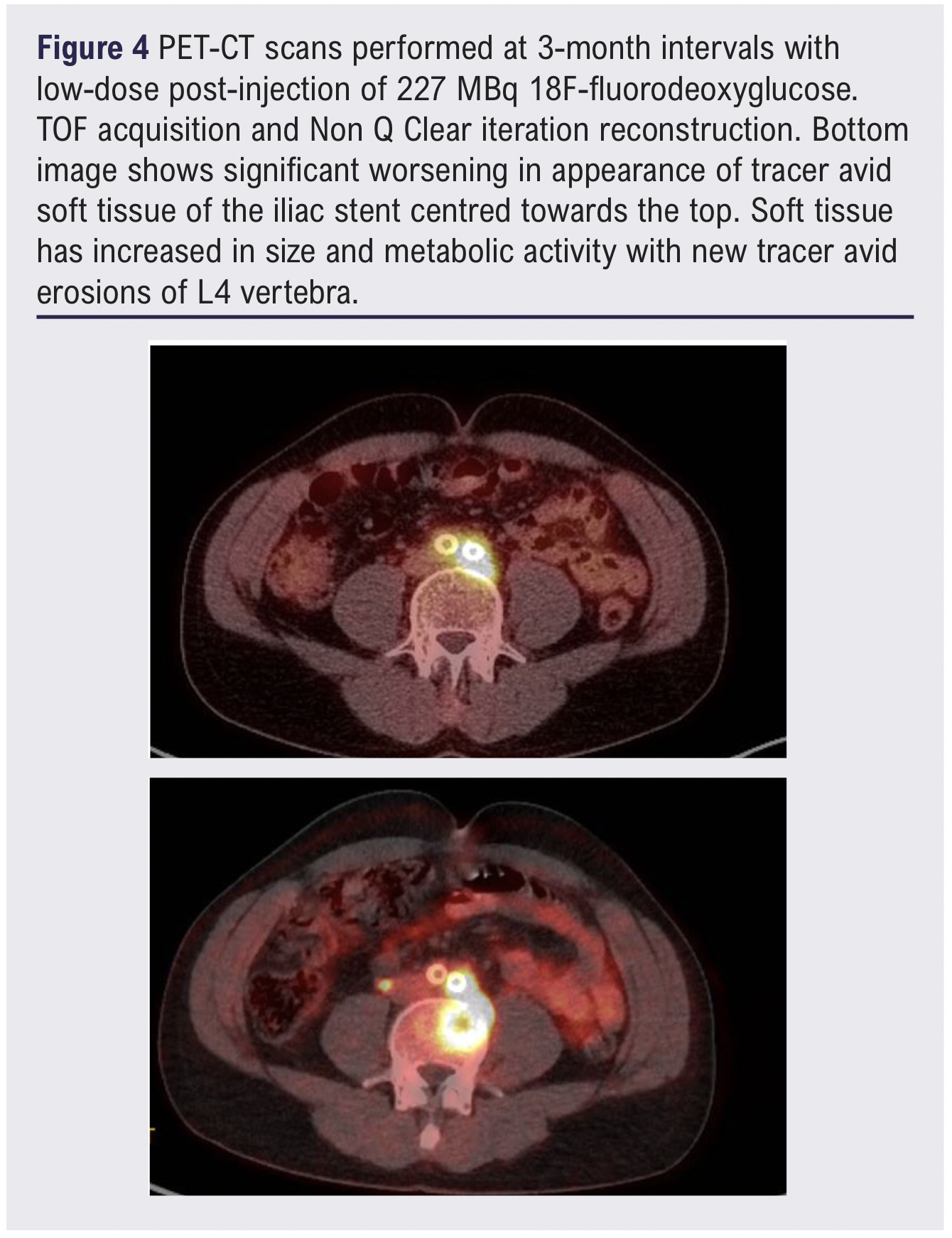
After several weeks of treatment, repeat blood cultures turned negative, providing initial reassurance. However, the patient’s back pain not only persisted but worsened, prompting a follow-up PET-CT scan. This revealed further increased FDG uptake with new evidence of bony erosion involving the L4 vertebral body (SUVmax increased to 15.6) (Figure 4). These findings were re-discussed at the MDT meeting and the decision was made to explant the iliac stents and reconstruct the aortoiliac system.
The iliac stents were completely explanted and aortic reconstruction was performed using a bifurcated graft fashioned from a 10 × 16 cm bovine pericardial patch (XenoSure® bovine pericardial patch; LeMaitre Vascular, Burlington, Massachusetts, USA). The patch was divided into two strips, each fashioned into a tube graft using 5/0 Prolene sutures. All vascular anastomoses were constructed in an end-to-end fashion.
Intraoperative findings showed extensive inflammatory tissue enveloping the aortic bifurcation, with clear evidence of L4 vertebral body erosion. The left ureter was found adherent to the inflamed tissue and carefully dissected free. Specimens from the perispinal tissue, anterior wall of the left common iliac artery and the explanted stents were sent for histopathology and microbiological analysis. All samples grew S. epidermidis, consistent with prior blood culture results. Additionally, histological evaluation confirmed osteomyelitis involving the L4 and L5 vertebrae. The case was discussed with the infectious diseases team who recommended a prolonged course of antibiotics and interval follow-up with MRI of the lumbar spine.
Serial follow-up MRI scans of the lumbar spine initially demonstrated L4–L5 discitis and osteomyelitis, with marked destructive changes involving the L5 vertebral body and an associated prevertebral fluid collection 26 x 19 mm. The patient was treated with a prolonged course of intravenous antibiotics as per local microbiology recommendations, and subsequent imaging showed regressive radiological features with resolution of inflammatory changes and a significant reduction in the size of the prevertebral collection (Figure 5).
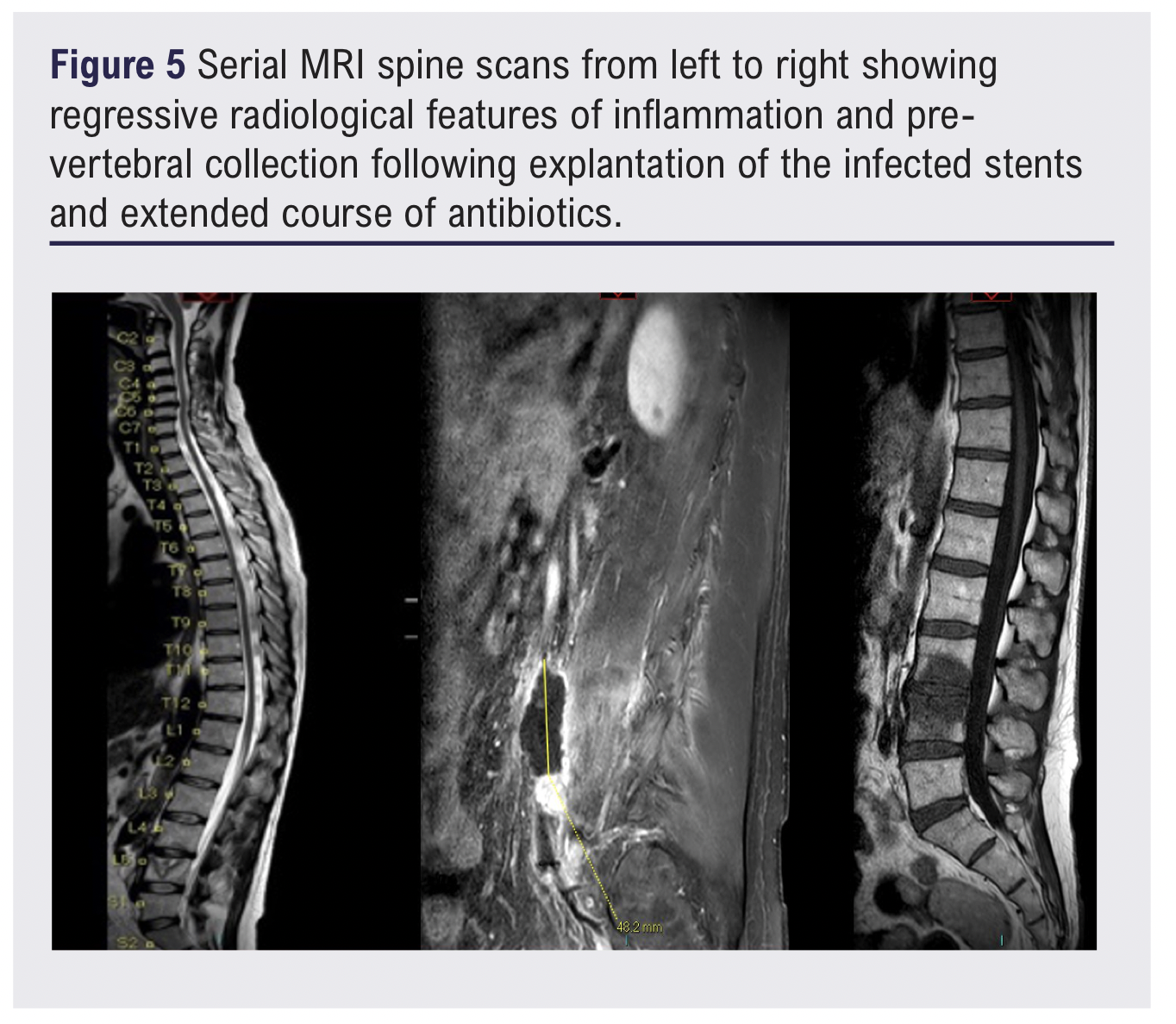
The patient was followed up for 6 months and showed good recovery, no symptoms or signs of infection and improved quality of life in terms of walking distance. However, the patient reported having retrograde ejaculation, which is one of the known complications of open aortic surgery, and has been referred to urology.
Discussion
The most common complications following endovascular treatment of AIOD are iatrogenic in nature, including vascular access site issues, arterial perforation, dissection and distal embolisation. Septic complications, on the other hand, are rare.2 While the clinical course and management of prosthetic bypass graft infections are well documented, infections involving endovascular stents remain poorly characterised due to their rarity. Nonetheless, published reports suggest that stent infections carry a substantial burden of morbidity and mortality.3 To our knowledge, this is the first documented infection of a VIABAHN VBX Balloon Expandable Endoprosthesis complicated by contiguous vertebral discitis. The unusually rapid onset – within one week of implantation – in an immunocompromised patient highlights the aggressive potential of this condition.
Although the overall incidence of stent graft infections is estimated to be less than 1%, when they do occur, these infections can result in catastrophic outcomes. Perioperative 30-day morbidity and mortality rates in the setting of stent graft infections have been reported at approximately 35% and 11%, respectively.4 Furthermore, within the first year postoperatively, the rates of graft-related complications and reinfection can exceed 10% and 5%, respectively. As such, prompt removal of the infected prosthesis and implementation of preventive measures are essential for improving outcomes. While most cases are caused by Staphylococcus species, infections due to Salmonella have been associated with particularly poor prognoses.5
Stent graft infections typically present with non-specific systemic symptoms such as fever, chills, malaise, fatigue and more localised signs including abdominal or back pain. CT imaging plays a central role, with typical findings including periaortic fluid collections, intraluminal or periaortic gas, soft tissue stranding and disruption of the aortic wall or aneurysm sac continuity.6 Early imaging was decisive. PET-CT quantified metabolic activity with SUVmax values rising from 10.6 to 15.6, correlating with clinical deterioration and MRI evidence of a 26 × 19 mm prevertebral abscess. Such quantitative metrics are valuable for monitoring disease progression and guiding timing of surgery.
Smeds et al highlighted that the optimal management of infected stent grafts is explantation followed by reconstruction using autogenous tissue, owing to its superior resistance to reinfection.4 When autologous reconstruction is not feasible, antibiotic-impregnated prosthetic grafts may serve as an acceptable alternative. Similarly, Chaufour et al demonstrated that complete explantation of the infected endograft remains the most effective strategy, often resulting in full resolution of infection and reducing the need for reintervention.6 This evidence-based approach was adopted in our case, with favourable clinical and radiological outcomes following surgical explantation and antibiotic therapy.
Despite technological advancements, stent infections remain a rare but serious complication. The most commonly implicated pathogen is Staphylococcus aureus, with remote bacteraemia being a significant risk factor—often associated with indwelling intravascular catheters, restenosis angioplasty or intravenous therapies.7 Animal models have demonstrated that balloon-expandable stents are particularly susceptible to infection when challenged intravenously with S. aureus within three weeks post-deployment.8 In addition, S. epidermidis has been identified as a causative organism, likely introduced during the procedure itself.9
Infections involving iliac stents, though infrequent, represent serious complications with significant associated morbidity and mortality.10 These infections have been documented in both bare metal and covered stents.11 Contributing risk factors include poor aseptic technique, absence of perioperative antibiotic prophylaxis, and underlying patient comorbidities.12 Accurate diagnosis often relies on a high index of suspicion, supported by imaging modalities such as CT or PET-CT scans.10
The VIABAHN VBX Balloon Expandable Stent has emerged as a reliable device in the treatment of iliac artery disease, demonstrating strong clinical outcomes. Studies comparing it with the Gore Iliac Branch Endoprosthesis indicate similar safety and effectiveness, with the VBX offering added benefits such as increased flexibility in branch configurations and a lower incidence of endoleaks. Its favourable properties – including high conformability – support its use in complex anatomy.13 The VBX device consists of a stainless steel balloon-expandable frame covered by expanded polytetrafluoroethylene (ePTFE). While ePTFE provides excellent conformability and sealing properties, its microporous structure may allow bacterial adherence and biofilm formation when exposed to transient bacteraemia. The patient’s prior lymphoma and chemotherapy likely resulted in prolonged B-cell depletion and impaired immune response, further predisposing to early infection.
Definitive management of iliac stent infections typically necessitates surgical removal of the infected prosthesis followed by arterial reconstruction, often employing autologous vein grafts.14 Antibiotic therapy alone is rarely curative in these cases and is usually insufficient without surgical intervention.11 Mortality rates associated with infected stent explantation have been reported to be between 12.5% and 32.5%, highlighting the potentially life-threatening nature of this condition.10,11
Conclusion
Endovascular therapy for AIOD is minimally invasive and has favourable short-term outcomes. Stent-related infections are an extremely rare but potentially devastating complication. This case highlights the diagnostic challenges and severe sequelae of iliac stent infection, including vertebral osteomyelitis and discitis, despite the use of modern covered balloon-expandable devices such as the VIABAHN VBX Endoprosthesis. Prompt diagnosis through multimodal imaging, microbiological confirmation and coordinated multidisciplinary decision-making were critical to achieving a favourable outcome in this patient. Ultimately, definitive management required surgical explantation, aortoiliac reconstruction, prolonged antimicrobial therapy and radiological surveillance. As the use of complex endovascular devices increases, clinicians must maintain a high index of suspicion for infection in the presence of unexplained systemic or localised symptoms post-intervention. This case underscores the importance of vigilance, early imaging and timely intervention to mitigate rare but life-threatening complications.
Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2026;ONLINE AHEAD OF PUBLICATION
Publication date:
February 12, 2026
Author Affiliations:
1. Liverpool Vascular and Endovascular Service, Liverpool, UK
2. School of Medicine, Leeds University, Leeds, UK
3. School of Physical Sciences, University of Liverpool, Liverpool, UK
4. Liverpool Cardiovascular Service, Liverpool, UK
Corresponding author:
Mina Abdelmalak
Liverpool Vascular and Endovascular Services, Aintree University Hospital, Lower Lane, Liverpool
L9 7AL, UK
Email: [email protected]











