ORIGINAL RESEARCH
Perception and acceptability of open versus endovascular treatment of common femoral artery disease: barriers and facilitators for randomised controlled trials
Kaneta G,1 Saratzis A,2 Zayed H1
Plain English Summary
Why we undertook the work: Many patients have reduced blood supply to the legs due to narrowed or blocked blood vessels (arteries). Minimally invasive keyhole (endovascular) interventions are often used to improve the blood supply by opening up these diseased arteries. There is some controversy about whether these keyhole interventions should be used in the main artery in the groin, which is usually treated with open surgery. This study aimed to assess opinions about undertaking a trial to compare open and keyhole surgery on this main artery in the groin.
What we did: We conducted an online survey and face-to-face interviews with doctors who regularly use both of these treatments in patients with diseased arteries. We asked whether they thought a trial was needed, whether they would be willing to participate in such a trial, and what they considered to be the most important factors that would obstruct or facilitate such a trial.
What we found: Most people think a trial is needed and would be happy to participate. Several important factors were identified which would need to be addressed to ensure such a trial could be successfully delivered.
What this means: There is enthusiasm and a willingness to undertake a trial to compare open and keyhole surgery on this main artery in the groin. Potential obstructing or facilitating factors have been identified, which will require careful consideration and attention when designing and delivering such a trial.
Abstract
Introduction: The common femoral artery (CFA) is often affected by atherosclerosis in patients with peripheral arterial disease, requiring revascularisation. Open surgical CFA endarterectomy (CFAE) remains the standard of care in this context; however, there have been multiple recent advances in endovascular CFA therapies. However, randomised controlled trials (RCTs) comparing CFA treatments have suffered from multiple pitfalls. This research aimed to assess opinions regarding potential barriers and enablers of delivering a high-quality RCT of open surgical CFAE versus endovascular CFA therapy.
Methods: A mixed-methods qualitative study was performed, including a structured online survey and face-to-face semi-structured interviews with healthcare professionals. Survey content and interview topic guides were developed following a literature review to identify ongoing and completed RCTs comparing CFA treatments. The data were analysed using thematic analysis.
Results: The online survey was completed by 121 participants, including vascular surgeons (n=75, 62%) and interventional radiologists (n=22, 18%), mostly from the UK (n=92, 76%). A total of 61 participants (51%) would be willing to take part in an RCT comparing open versus endovascular CFA revascularisation. The majority (n=89, 74%) believed that such an RCT is urgently needed. Fifteen participants were interviewed face-to-face. Five main themes emerged regarding barriers and facilitators for a high-quality RCT in this context: factors directly limiting patient recruitment; clinicians’ attitudes towards equipoise between treatments; clinicians’ attitudes towards endovascular therapies; attitudes towards outcomes examined in a potential RCT; and factors facilitating patient recruitment. From these, 10 sub-themes were identified.
Conclusion: The majority of survey respondents believed an RCT comparing open and endovascular CFA revascularisation is necessary and would participate in such a trial. Important barriers and enablers, grouped in five overarching themes, have been identified, which would require serious consideration when designing and delivering such an RCT.
Introduction
The standard of care for the treatment of atherosclerotic disease of the common femoral artery (CFA) remains open surgical CFA endarterectomy (CFAE). Endovascular interventions, however, have become first-line therapies for atherosclerotic disease of other arterial segments. This is due to their minimally invasive nature, low rates of perioperative complications and patient preference.1–4 High rates of technical success and low rates of complications with endovascular management of CFA stenosis have been reported; one small randomised controlled trial (RCT) suggests that short-term (30 days) outcomes might be better after CFA angioplasty than CFAE.1,5,6 This RCT, however, was underpowered to detect differences in clinical outcomes such as amputation-free survival. Furthermore, this RCT and other similar attempts in this clinical context have suffered from low recruitment rates and limited follow-up. High-quality large-scale trials are urgently required to assess the clinical effectiveness of CFA endovascular procedures, which are now common in clinical practice.7
As previous RCTs in this area have suffered from multiple issues, the experiences of healthcare professionals need to be investigated and explored in a structured manner to understand potential barriers and enablers in RCT delivery.
This research aimed to use established qualitative methodology to understand issues surrounding RCT delivery in the context of comparing the effectiveness of surgical versus endovascular CFA revascularisation.
Methods
Regulatory approvals
The study was approved by the London Bromley National Health Service (NHS) Review Ethics Committee (REC) in December 2019 (reference number: 20/LO/0059). It was approved by the United Kingdom (UK) Health Research Authority (HRA) (reference number: 274726).
Informing the content of the survey and topic guides
A systematic literature review using the Medline and Embase databases (since inception) in December 2019 (updated March 2020) was undertaken to identify all studies comparing endovascular and open surgical CFA revascularisation for atherosclerotic disease. The following terms were used: (“peripheral arterial disease” OR “peripheral artery disease”) AND “common femoral” AND (“endovascular” OR “surgery”) – any language. The clinicaltrials.gov and ISRCT registries were also searched (December 2019) using the same terms to identify ongoing studies. We identified 39 published studies (randomised and non-randomised) relevant to CFA treatments and one ongoing RCT, which were then reviewed to identify potential barriers and facilitators in delivering randomised research. The survey content and interview topic guides were then composed. Both were reviewed by three independent vascular surgeons and two vascular interventional radiologists. A participant information sheet was prepared and sent to all those taking part, summarising the hypothesis and aims.
Online survey
A structured online survey with both closed and open questions was developed by the two senior investigators between December 2019 and March 2020 (Appendix 1 – online at www.jvsgbi.com). The survey link was disseminated via email between March and May 2020 to the membership of the Research Collaborative for Peripheral Arterial Disease (RCPAD) and the Vascular and Endovascular Research Network (VERN), representing 894 cardiovascular professionals across 53 countries. In addition, existing RCPAD and VERN social media accounts (Twitter) with a total followership of 4114 at the time of dissemination were used to further publicise the survey with weekly tweets between March and May 2020. The three authors also disseminated the survey link in their departments and professional networks during the same period. The survey could only be filled in once per Internet Protocol (IP) address requiring the practitioner to use a unique direct link, to avoid duplicate entries. Survey entries were also screened for duplicates upon completion.
Semi-structured interviews
Semi-structured qualitative interviews were performed between January 2021 and September 2021 (face-to-face). The interviews were conducted once the analysis of the online survey had been completed so that the topic guide was written accordingly (Appendix 2 – online at www.jvsgbi.com). The interview sample recruited consisted of healthcare professionals who provide primary or secondary care to patients with peripheral arterial disease and who could provide informed consent and attend the interview. They were recruited via the online survey (asked to provide their contact details) or directly by the three investigators in person, across seven different secondary care institutions in the UK, Greece and Italy. Informed consent was obtained in all cases. We used open-ended questions framed in a locally and culturally appropriate context to encourage discussion and exploration of pertinent barriers and facilitators. Interviews were recorded (audio) and transcribed (where necessary) within 12 hours. Topic guides for future interviews were adjusted accordingly, based on prior topics explored, to ensure all barriers and facilitators were explored (iterative design).
Analysis of data
The categorical data from the online survey were summarised in a spreadsheet. The replies to open-ended free text questions were checked for sanity and accuracy, duplicates were removed, and the text was then analysed as per the procedure described below.
For the interviews, the primary data source was the recorded audio file and (where necessary) the transcript for each interview. The text from both the online survey and transcribed interviews was coded using content analysis procedures. Transcription and coding were completed within one day of each interview. Each audio file and transcript was independently coded. A master-sheet codebook with themes and quotes was then created; the two investigators met to discuss themes after each set of interviews was analysed as quickly as possible. If the agreement in themes was less than 90%, another investigator would adjudicate accordingly. Themes were derived from a combination of pre-set questions in the topic guide as well as from data in the transcript of each interview. At this stage, the free text of the online survey was also analysed to identify emerging themes. Transcripts were then recoded using the final version of the interview and survey master-sheet/codebook. A summary of all the themes was generated; similar themes were grouped into broad and abstract categories. Qualitative data analysis software (NVivo 9, QSR International) was used to tabulate theme frequency using the master-sheet/codebook count and sort the minor themes.
Results
Online survey
A total of 121 individual participants from 69 vascular units completed the online survey. Most of the units were in the UK (n=92, 76%), followed by other European countries (n=13, 11%) including Italy, Germany, Greece and Belgium. Participants were mainly consultant (“attending”) vascular surgeons (n=69, 57%), followed by vascular surgery trainees (n=24, 20%) and interventional radiology consultants (n=22, 18%). Most participants had more than 5 years of experience as independent practitioners (n=57, 47%) following completion of their specialist training (Table 1).
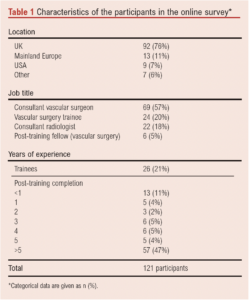
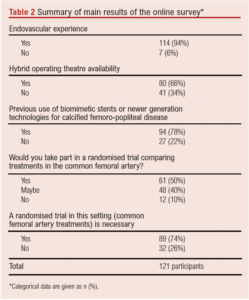
A total of 114 participants (94%) performed endovascular and hybrid vascular procedures at least once weekly; 80 participants (66%) had access to a hybrid operating theatre.
A total of 61 participants (51%) would be willing to take part in an RCT comparing CFA stenting versus CFAE for CFA disease. The majority (n=89, 74%) believed an RCT of best endovascular therapy against best open surgery for CFA lesions is needed (Table 2).
Semi-structured interviews
Overall, 44 healthcare professionals were invited to take part in the interviews, of which 15 participants were interviewed face-to-face, including six vascular surgeons, six radiologists and three specialist vascular nurses. All worked in secondary care (hospital) settings, had experience of treating patients with peripheral arterial disease and had recruited at least one patient in an RCT in the last two years.
Five main themes emerged regarding barriers and facilitators for an RCT comparing open versus endovascular treatments for CFA steno-occlusive disease: (1) factors limiting patient recruitment (barriers); (2) attitudes towards equipoise between treatments (arms); (3) attitudes towards endovascular therapies; (4) attitudes towards outcomes examined in a trial; and (5) factors facilitating patient recruitment. From these, 10 subthemes were identified. Saturation was reached in this analysis. A thematic map is provided in Figure 1.
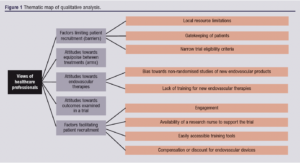
Factors limiting patient recruitment (barriers)
Local resource limitations: Limitations relating to time, space for screening, consenting, follow-up as well as completion of data collection forms were noted. With regard to time, competing clinical priorities and both academic and clinical bureaucratic workload were the most common issues. With regard to space, no availability of rooms in the usual clinic environments or the vascular wards were noted as the main limiting factors. A further common limitation noted in all interviews was the lack of dedicated funding for research nursing time in the participants’ units.
[Quotes] “… there is not time before or after my clinic to screen patients for any study, let alone something as complex as a randomised trial…”, “… I have not time to discuss concepts such as randomisation during regular clinics on most days…”, “… we never see any of the funds allocated to these studies come to our department, especially in the form of research nurse salary…”.
Gatekeeping of patients: Some healthcare professionals noted that bias towards pre-selecting or excluding certain patients are common in this context. This included avoiding contacting patients with severe ulcerations in their lower limbs, patients with extensive disease of their arteries, those who were very immobile, and patients with potentially difficult personalities. It was noted that they would not want to recruit multi-morbid patients as they did not want to burden them with additional assessments or follow-up visits.
[Quotes] “… I wouldn’t select a case where I have to do extensive endarterectomies and then stent inflow or outflow on top, as I think it would “mess” up the study results…”, “… I’m not keen on talking to patients who can’t walk for such a trial; I don’t think these patients would do the study right as it would make the other arm look much better in the long term…”, “… I really don’t want to recruit patients who have several health problems or might be near the end of their life to a study which adds extra burdens such as more hospital visits; I can’t see the point…”.Narrow trial eligibility criteria: Strict or very descriptive eligibility criteria in terms of both symptomatology and anatomy (ie, number of arteries occluded and/or stenosed) were universally viewed as a key barrier to successful recruitment. There was clear consensus against such an approach.
[Quotes] “… The main problem with all of the femoro-popliteal trials is always the very long list of exclusion criteria; it makes it impossible to find these patients…”, “… This study will have to include “all-comers”, otherwise no one will feel happy to take part, not patients and definitely not healthcare staff…”.
Attitudes towards equipoise between treatments (arms)
All healthcare professionals discussed equipoise during the interviews. Some expressed “no equipoise” between CFAS versus CFAE for CFA disease (“… there is no reason to invest in any such study as CFAE is an established technique and no one will ever randomise a patient…”). Others expressed that they view both treatments as identical (“… in the right hands, open or endo – doesn’t really matter, as long as you pick the right patients and you have the experience…”). There was unanimous agreement that new therapies, especially stents, must be urgently assessed in randomised trials in terms of their role in treating CFA disease. Overall, we found that views with regard to equipoise varied widely, even though most (if not all) professionals do agree that randomised research is urgently needed in this domain. A common sub-theme which emerged was the lack of evidence specifically relating to stenting the CFA; radiologists and surgeons often expressed that they have been against using stents in this region specifically because there is no high-quality evidence assessing their use for this artery. It was found that this might be a driver towards recruitment in such a study.
Attitudes towards endovascular therapies
Bias towards non-randomised studies of new endovascular products: Several healthcare professionals reported strong bias towards recruiting patients in non-randomised studies (eg, registries) of new endovascular products. This was felt to be a common practice in vascular units nationally and internationally. It was felt that this stops clinicians from promoting pragmatic trials comparing treatments head-to-head, especially when this is publically funded research that does not generate considerable income. Consultant surgeons and radiologists noted that certain individuals “are only interested in their own studies” or favour “studies which generate income for the departments”.
Lack of training for new endovascular therapies: Most healthcare professionals expressed concerns over a lack of uniform training amongst centres taking part in such trials. Another theme that emerged was related to the experience of the operators. Many found that this would be a source of bias when recruiting new centres and when analysing results. Finally, lack of adjudication with regard to how the endovascular treatments have been used was another theme which often emerged as a barrier in terms of delivering a successful trial which would be translated to clinical care.
[Quote] “… we would like to take part in such a study but we have had no formal training on how to use things like atherectomy or lithotripsy in a CFA. How are we going to be able to do the study in my department …?”, “… we need to ensure that all operators in the study have experience in using endovascular equipment developed for CFA disease. I think this will be very difficult to achieve…”, “… a core-lab or some form of quality control is needed in the endovascular arm. Different people use endovascular devices in a different way. This has to be standardised or at least one has to know how the endovascular arm was treated…”.
Availability of endovascular therapies: Professionals noted that some endovascular therapies might not be available in their centre. This was seen as a key barrier to opening their site as a recruitment centre: “… there is no point discussing taking part in a study if my department does not have access to most of the endovascular tools used in endo-heavy hospitals…” or “… we need to buy the endovascular equipment first, before we take part in any trial in this area…”.
Attitudes towards outcomes examined in a trial
The main theme emerging in this area related to the use of clinical and cost-effectiveness outcomes – namely survival, amputation and healthcare costs – as part of a primary outcome measure in a successful RCT. Interestingly, several individuals expressed a view that a successful trial would have to include patients with intermittent claudication; the main outcome of interest should be walking distance for this specific sub-population (and not amputation or patency driven). Overall, quality of life assessments were noted as a key outcome, to be included as a secondary outcome measure in any trial in this disease area, both for those with chronic limb-threatening ischaemia and those with intermittent claudication. At the same time it was noted that patient-reported outcomes relating to quality of life are not easy to use or widely validated for patients with peripheral arterial disease, especially limb-threatening ischaemia. There was a common theme against using patency of the treated lesion as the main outcome of interest, regardless of the presenting symptomatology of the trial population.
Factors facilitating patient recruitment
Engagement: It was deemed important for successful trial recruitment to maximise engagement/frequent interaction with sites (“… the main study site needs to keep in touch with all sites very regularly…”). This includes meetings face-to-face and remote availability most days of the week, with the lead site(s) taking part in these activities. Regular site visits were also noted by most professionals as a key facilitator as per their experience. It was viewed, especially by the nursing staff, that the doctors must engage with the specialist and ward nurses daily to ensure patients eligible for recruitment will be identified.
Overall, this theme highlights the importance of maximising engagement, including face-to-face meetings with all interested parties and maintaining regular visits to sites.
Availability of a research nurse to support the trial: A common theme was the availability of a research nurse (or other relevant staff) on site to help with screening and study processes: “… the patients are often seen in clinic or the ward interchangeably. We need access to a dedicated nurse or some form of individual who will be chasing study procedures almost daily. This is where we keep failing with peripheral arterial disease trials …”
Easily accessible training tools: Online rather than paper-based training was a common theme emerging with regard to trial procedures. Also, many commented that a module for the endovascular therapies should be made available remotely, as certain clinicians might not have received the same type of training for each endovascular device assessed in a potential trial.
Compensation or discount for endovascular devices: Consultant radiologists and surgeons noted that discounts for using the endovascular devices as part of such a trial by the producers would “… definitely support recruitment …” or “… change the minds of our managers about not taking part in similar trials …”.
Discussion
Recent advances in endovascular procedures for atherosclerotic CFA disease now allow the treatment of complex anatomies. At the same time, there is no large RCT comparing the clinical and cost-effectiveness of open versus endovascular revascularisation of the CFA. This may be due to a variety of reasons. Delivering successful RCTs to address this important clinical question necessitates an in-depth understanding of relevant barriers and facilitators.
To our knowledge, this is the first study to systematically examine the perceptions and assess the opinions of healthcare professionals to successfully delivering a high-quality RCT comparing CFAE versus CFA stenting.
This study showed that most vascular interventionists surveyed believed that such an RCT is necessary and would be willing to potentially recruit patients. At the same time, the study identified several potential barriers, and several facilitators, which require serious consideration during the design and delivery of any such future RCT.
CFAE remains the gold standard treatment for significant CFA disease because it has been proven to be safe, durable, with high technical success rates.8 However, CFAE is associated with potential perioperative morbidities such as bleeding, wound infection, and re-intervention and short-term morbidity and mortality rates as high as 15% have been reported.9 Of note, CFAE is mostly performed under general anaesthesia, which presents an added risk in old frail patients.1-4 Additionally, CFAE could be technically challenging in obese patients and those with previous groin surgery or radiotherapy. These factors, in addition to the minimally invasive nature of endovascular techniques and the significant advancements in this field, make CFA angioplasty with/without stenting an appealing alternative approach to treat CFA disease. However, endovascular techniques in this context are generally burdened by the added costs and the frequent need for re-interventions.5,6,10,11 There are no previous high-quality randomised studies which provide enough information on the performance of stents or other newer endovascular treatments in this challenging anatomical location.7
Previous attempts to conduct RCTs have encountered difficulties perhaps because they were designed and delivered without formal assessment of the perception of such trials. A small randomised study tried to address this controversy; however, recruitment was found to be a challenge (117 patients over 30-month period). Additionally, this study had strict inclusion and exclusion criteria which do not necessarily reflect the day-to-day practice of most vascular units. There was also a lack of long-term clinical outcome data such as amputation-free survival.6
Detailed exploration of research delivery in terms of barriers and enablers has been strongly recommended prior to designing and delivering successful large-scale RCTs.12,13 Our study cohort reflected the diverse background of vascular interventionists treating CFA lesions, including vascular surgeons and interventional radiologists, with varying levels of expertise (79% were consultants with almost half of them having more than 5 years’ experience of independent practice). Overall, 94% of those surveyed had the necessary open and endovascular skills to deliver both CFAE and CFA endovascular treatments (eg, stenting), with 66% having access to hybrid operating theatres. In particular, 78% were familiar with new devices used in the femoro-popliteal segment, including biomimetic stents. On the one hand, this shows that the necessary skill set and operating environment to deliver both treatment modalities for CFA disease are available in most vascular centres included in this study. On the other hand, almost a quarter of those surveyed indicated that further endovascular training would still be required to deliver an RCT. Although this should be considered as a potential barrier, this is a better starting position compared with other procedures at the time of their introduction to daily practice, such as endovascular aneurysm repair where initially the necessary skills were limited to certain centres prior to testing in an RCT.14
Furthermore, as part of this study, interviews were conducted with 15 healthcare professionals (surgeons, radiologists and nurses). The thematic analysis identified various barriers – for example, lack of research infrastructure in certain institutions (including research nurses or allocated research time), lack of certain endovascular devices or endovascular training, as well as the prohibitive costs of advanced endovascular tools. Extensive exclusion criteria were identified as an important factor which limits recruitment in similar trials. We have grouped the emerging themes into five groups with 11 sub-themes. This will help future researchers to design a trial with a much greater chance of successful delivery.
Regarding potential study facilitators, there was an agreement that obtaining the endovascular devices at a reduced cost or free of charge (as part of a costed study) would greatly facilitate recruitment. Additionally, appropriate costing for and provision of the necessary research infrastructure to support the trial (such as research nurses and clinicians’ time) would also help recruitment.
In terms of study design, there was a recurring theme that the outcome measurements should include clinically relevant metrics such as amputation-free survival, limb salvage and quality of life, especially if patients with claudication were included. It was also felt that the usual outcome measures such as patency rate should not be the only endpoint for this trial. These findings should be taken into serious consideration when planning, designing and delivering an RCT in this area.
This study has some limitations. As the healthcare professionals surveyed in this study came from different countries, this might have induced heterogeneity of the feedback based on the experience from various healthcare systems with different reimbursement systems. Patients’ views are not included in this comprehensive analysis as it was beyond the scope of this current study which aimed specifically to specifically explore the perceptions of healthcare staff. However, we would strongly advocate including patients when designing any form of RCT in this setting.
Conclusion
This study showed that the majority of the surveyed healthcare professionals believe that an RCT comparing cost and clinical effectiveness of surgery versus endovascular treatments for CFA disease is necessary and the majority would be willing to recruit participants. It has also identified potential barriers and enablers which should be taken into consideration when designing and delivering such a trial.

Article DOI:
Journal Reference:
J.Vasc.Soc.G.B.Irel. 2023;2(4):215-221
Publication date:
July 21, 2023
Author Affiliations:
1. Guy’s and St Thomas’ NHS Foundation Trust, London, UK
2. Department of Cardiovascular Sciences, University of Leicester, Leicester, UK
Corresponding author:
Gabriela Kaneta
Research Fellow, Department of Vascular Surgery, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, UK
Email: gabriela.kaneta@ gstt.nhs.uk











